Abstract
Objectives: To assess the frequency of adverse effects among pediatric and adult patients and the clinical variables associated with a higher probability of developing side effects.
Methods: This retrospective study enrolled pediatric and adult patients who underwent Vagus nerve stimulation (VNS) implantation at our institution and had documented follow-up during clinic visits for at least 6 months after implantation. Data collected included demographic information, epilepsy diagnosis, and device data.
Results: A total of 43 patients with drug-resistant epilepsy who received a VNS device at our institution were enrolled. The median follow-up period was 12 months. Fourteen patients (32.55%) reported no side effects from VNS therapy. Side effects ranged from mild to severe, with significant side effects observed in 8 patients. Data on therapy efficacy were collected, and 10 patients (23.26%) reported no change in seizure frequency following device implantation.
Conclusion: This study demonstrates that VNS is an important adjunct treatment option for epilepsy patients. Dysphagia and dyspnea can be significant adverse effects leading to treatment discontinuation, aspiration pneumonia, intensive care unit (ICU) admission, and prolonged hospital stay. These effects are more frequent in patients with symptomatic generalized epilepsy, global developmental delay at baseline, previous ICU admissions, abnormal brain magnetic resonance imaging findings, and seizures with multiple semiologies.
Vagus nerve stimulation (VNS) therapy is a treatment modality for medically refractory epilepsy in patients who are not candidates for resective epilepsy surgery.1 Medically intractable epilepsy is defined by the International League Against Epilepsy (ILAE) as the failure of 2 properly chosen anti-seizure medications, at appropriate doses, to control seizures. Approximately 28–35% of patients with epilepsy continue to experience intractable seizures despite optimal medical therapy.2,3 Studies have shown that VNS therapy is effective in reducing seizure frequency. After 3 months of therapy, the intervention and treatment groups demonstrated a higher efficacy (24.5%) than the control group (6.1%) that received sham stimulation. Subsequently, the efficacy was compared between high and low VNS stimulation parameters, revealing better seizure control in the high-parameter stimulation group.4 In another series of patients, the reduction was 28% in the treatment group and 15% in the control group.5 Furthermore, VNS therapy is more effective over time after implantation, resulting in reduced seizures during long-term follow-up compared to patients treated with the best available medical therapy.6
For patients who are not candidates for epilepsy surgery, continued VNS therapy generally leads to a median seizure reduction of 17–55%, with a small percentage of patients (approximately 8.2%) achieving long-term seizure freedom.7 However, VNS therapy can cause side effects such as hoarseness, cough, shortness of breath, sore throat, neck pain, dysphagia, headaches, nausea, and vomiting.8 It may have disabling effects, particularly on respiration and swallowing.9,10 Additionally, VNS therapy can induce sleep breathing disorders, commonly obstructive sleep apnea, and a reduction in oxygen saturation during VNS stimulation.11-14
This study aimed to assess the frequency of adverse effects among pediatric and adult patients, as well as the clinical variables associated with an increased probability of developing side effects. The aim is to identify patients at higher risk of experiencing disabling adverse effects following VNS implantation, providing valuable information for medical decision-making and patient counseling before the procedure.
Methods
We conducted a retrospective analysis of pediatric and adult patients who underwent VNS implantation at our institution. The study received ethical approval from the Institutional Review Board. This descriptive retrospective study involved reviewing the medical records of patients diagnosed, treated, and followed at our epilepsy center located at King Faisal Specialist Hospital and Research Center (KFSH&RC) in Saudi Arabia.
The inclusion criteria comprised patients aged 1–60 years with generalized and focal epilepsy who had received a VNS device implantation at KFSH&RC and were subsequently followed for monitoring of VNS therapy and device programming. We conducted a retrospective review of side effects based on documented follow-up records at least 6 months after the implantation procedure.
The study included 43 patients who underwent VNS implantation between March 25, 2021, and May 30, 2022. Collected data encompassed demographic information and epilepsy-related characteristics such as age, sex, epilepsy type, etiology of epilepsy, seizure semiology, age at seizure onset, duration of epilepsy prior to VNS implantation, previous admission to the intensive care unit (ICU), presence of swallowing or respiratory difficulties, number of anti-seizure medications, comorbid conditions, history of epilepsy surgery, and treatment efficacy. Brain magnetic resonance imaging (MRI) and electroencephalogram (EEG) results were also documented. Furthermore, we collected device-related data, including the device model, as well as device settings such as current output, pulse width, frequency, device on-time (in seconds), device off-time (in minutes), auto-stimulation (autostim) current, and magnet current.
Statistical analysis
Descriptive and inferential statistical analyses were performed on the collected data. The socio-demographic characteristics of the epilepsy patients, along with their VNS parameters and other categorical variables, were analyzed by calculating simple frequencies and percentages, which were then tabulated. To determine the significant associations between various risk factors and the severity of side effects and treatment outcomes of VNS, Chi-Square and Fisher’s Exact Test were employed. Statistical significance was set at a p-value of 0.05 or lower, with a 95% Confidence Interval. All statistical calculations were conducted using IBM SPSS Software, version 29.0.0.
Results
Demographic data
The study aimed to determine the effectiveness of VNS therapy for patients with epilepsy, as well as its associated side effects. Table 1 presents a comprehensive summary of the socio-demographic characteristics and other pertinent factors of the patients who received VNS therapy, comprising a total sample size of 43 individuals with a median age of 14 years.
- The Socio-demographic and other features of the patients who underwent VNS implantation (n=43).
The VNS Therapy efficacy
The effectiveness of VNS treatment was evaluated by measuring the reduction in seizure frequency. Out of the patients, 10 (23.3%) reported no improvement, 30 (69.8%) experienced a decrease in seizure frequency, and 3 (7.0%) attained complete seizure freedom.
Side effects
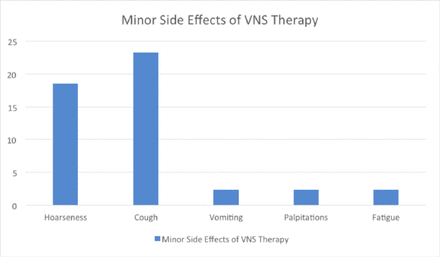
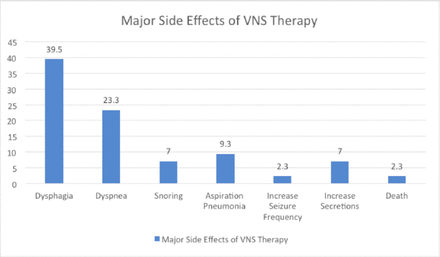
Regarding side effects, 29 patients (67.4%) reported experiencing side effects following VNS therapy, while 14 patients (32.6%) did not encounter any. It is important to note that patients may have experienced multiple side effects. Among those who experienced side effects, 8 patients (18.6%) reported severe side effects, while 35 patients (81.4%) did not encounter severe side effects. Furthermore, 6 patients (14.0%) necessitated admission to the ICU, whereas 37 patients (86.0%) did not require such admission. When analyzing the minor side effects of VNS therapy (Figure 1), the most commonly reported symptoms were hoarseness (18.6%) and cough (23.3%). Vomiting, palpitations, and fatigue were reported by only a small number of patients (ranging from 1 to 2.3%). Regarding the major side effects of VNS therapy (Figure 2), dysphagia was the most prevalent, affecting 17 patients (39.5%). Dyspnea was reported by ten patients (23.3%), snoring by three patients (7.0%), and aspiration pneumonia by four patients (9.3%). Other less common major side effects included increased seizure frequency, an increase in secretions, and even one reported case of death.
- Minor side effects of VNS therapy.
- Major side effects of VNS therapy.
Epilepsy-related characteristics
The median duration of epilepsy prior to VNS implantation was 12 years. Table 2 provides information on epilepsy-related characteristics and conducted investigations among patients who underwent VNS implantation. Regarding comorbidities, a significant portion of patients had additional conditions. Global developmental delay was observed in 20 patients (46.5%), cognitive impairment in 10 patients (23.3%), and autism, hearing loss, and blindness in one patient each (2.3% each). All 43 patients included in the study were equipped with the Sentiva 1000 device model for their VNS therapy. Table 3 outlines the device settings in normal mode, auto-stimulation mode, and the magnet stimulation parameters.
- Epilepsy-related features and investigations in patients who underwent vagus nerve stimulator (VNS) implantation (n=43).
- Device parameters.
Risk factors associated with severe side effects of VNS therapy
Risk factors associated with severe side effects of VNS therapy were examined in a sample of 43 patients. The first risk factor investigated was Global Developmental Delay (GDD). Among participants without GDD, 62.9% did not experience severe side effects, whereas only 12.5% of those with GDD were free from such effects. This difference demonstrated statistical significance (p=0.017), indicating that GDD is associated with an increased likelihood of severe side effects from VNS therapy. The next risk factor analyzed was seizure classification, dividing participants into those with focal seizures and those with generalized seizures.
Interestingly, none of the participants with focal seizures experienced severe side effects, while all 8 participants with generalized seizures did. Although the difference between the two groups did not reach statistical significance (p=0.171), this finding suggests a potential association between generalized seizures and an increased risk of severe side effects. The presence of previous ICU admissions was identified as a significant risk factor for severe side effects. Among participants without previous ICU admissions, 94.3% did not experience severe side effects, compared to 50% of those with severe side effects who has a history of previous ICU admissions (p=0.007). The MRI findings were examined as a potential risk factor as well. Participants were categorized into those with a normal MRI and those with an abnormal MRI. While all participants with an abnormal MRI experienced severe side effects, none of those with a Normal MRI did. Although the p-value (p=0.066) suggests a trend, the sample size may be too small to reach statistical significance. EEG Features were analyzed in terms of focal versus generalized and multifocal patterns. None of the participants with Focal EEG features had severe side effects, while all participants with generalized and multifocal EEG Features did. However, this difference did not reach statistical significance (p=0.171). Participants who had undergone different types of epilepsy surgeries were evaluated, and no severe side effects were reported in the specific surgery categories mentioned. However, 25% of corpus callostomy patients experienced severe side effects. Among the clinical variables statistically associated with improvement after VNS implantation, the absence of a history of epileptic spasms was significant (p=0.009).
Discussion
This study specifically focused on evaluating the adverse effects of VNS implantation. The patient population included both pediatric and adult patients, with a median age of 14 years. The classification of epilepsy in our patients was based on the ILAE definition of medically intractable epilepsy, with a median duration of approximately 12 years before VNS implantation.15
Previous studies have demonstrated that VNS is an effective adjunctive treatment for medically refractory epilepsy, particularly in cases where resective surgery is not feasible.7,16,17 The VNS therapy offers a tolerable treatment option that effectively improves the quality of life for patients.18,19 Approximately 30% of epilepsy patients are classified as medically intractable.20 In our study, approximately 79% (n=34) of patients had generalized epilepsy, for which VNS served as a palliative treatment option. For the remaining patients with focal epilepsy, VNS was employed as a palliative measure after epilepsy surgery failed to achieve optimal seizure control or when the seizure onset zone was located in the eloquent cortex. Epilepsy surgery was performed on 19 patients with focal and generalized epilepsy. The most frequent procedure was corpus callosotomy, conducted on 15 out of 19 patients (78.95%) to address atonic and tonic seizures. Other procedures, including functional hemispherectomy, frontal disconnection, posterior quadrant disconnection, and temporal lobectomy, were performed in one case each. The VNS was implanted after epilepsy surgery proved insufficient in adequately controlling seizures. The combination of epilepsy surgery followed by VNS might have contributed to higher rates of VNS therapy efficacy in our study population compared to the median seizure reduction reported in other studies.3,7 Regarding epilepsy etiology within our study population, 21 patients (48.8%) had an idiopathic etiology, followed by seven patients (16.2%) with a genetic etiology, as shown in Table 1 and Table 2. Epilepsy in the patients included in this study was often comorbid with cognitive impairment or GDD (n=30).
Adverse effects are anticipated in patients following VNS therapy; in most cases, these effects are mild and tolerable. Common adverse effects include cough and hoarseness resulting from stimulation of the recurrent laryngeal nerve, which causes vocal fold contraction at therapeutic stimulation parameters.21,22 In our series of patients, dysphagia was reported more frequently than in other studies, with 17 patients (39.5%) experiencing some difficulty swallowing after implantation. Another notable but less frequent adverse effect was dyspnea, reported by 10 patients (23.2%). These symptoms can significantly impact the quality of life and lead to challenges in feeding and therapy tolerance.
Additionally, 3 patients (6.9%) reported snoring, which can manifest as obstructive sleep apnea in patients with VNS. Adjusting the device settings, specifically by reducing the frequency of the stimulus and decreasing the duty cycle, can help alleviate symptoms of sleep apnea.8,11 None of the patients were referred for diagnostic polysomnography. However, it is advisable to screen for sleep-disordered breathing (SDB) and confirm the diagnosis through polysomnography to provide specific therapy, such as positive airway pressure, as the incidence of SDB tends to increase following VNS implantation.23,24 Interestingly, one patient (2.3%) experienced shortness of breath, which improved when the autostim mode was switched off without altering other device parameters. This approach may serve as a successful method prior to lowering the generator normal mode parameters.
Significant adverse effects were observed in 8 patients (18.6%), leading to swallowing assessment, adjustment of settings, device deactivation, or device removal. Within this group, seven patients had GDD, which was significantly associated with severe side effects (p=0.017), and 3 patients had previously undergone corpus callosotomy epilepsy surgery. Most patients had unknown etiology for epilepsy (n=5), while others had genetic etiology, meningoencephalitis, or anoxic ischemic encephalopathy. All patients had symptomatic generalized epilepsy with abnormal EEG findings indicating generalized and multifocal epileptiform discharges. The 8 patients had abnormal brain MRI results, including cerebral atrophy (3 patients), encephalomalacia (2 patients), heterotopia (2 patients), and schizencephaly (one patient). Four patients (50%) had a history of ICU admission due to seizures, and prior ICU admission was significantly associated with severe side effects (p=0.007). Adverse effects were severe enough to deactivate the device in four out of eight patients (50%), with one patient undergoing device removal and one patient experiencing complications leading to death, including aspiration pneumonia. These patients exhibited ≥2 seizure semiologies. The median device output was 1 mA, significantly lower than the typical therapeutic range of 1.5–3 mA,25,26 Notably, the device parameters did not contribute to the adverse effects as all patients were in the titration phase and had not yet reached optimal stimulation parameters.
Clinical characteristics of patients who developed severe side effects included GDD, symptomatic generalized epilepsy, multiple seizure semiologies, EEG findings of generalized and multifocal epileptiform discharges, abnormal brain MRI, and a history of prior ICU admission. These characteristics can aid in stratifying patients who may experience dysphagia and dyspnea after implantation, which can diminish the tolerability of VNS therapy and quality of life. It underscores the importance of screening for swallowing difficulties before and after implantation to detect and address such issues, thereby reducing the incidence of serious complications like aspiration pneumonia that may require ICU admission. Interestingly, non-invasive VNS through the auricular branch of the vagus nerve in epilepsy patients has shown a reduction in seizure frequency with side effects such as headaches, vertigo, nausea, dizziness, and fatigue, but dysphagia, dyspnea, and obstructive sleep apnea were not reported,27,28 Similarly, trials of non-invasive VNS at the neck for other indications like migraine did not report obstructive sleep apnea, dysphagia, or dyspnea.29,30
The implanted device in our study was the latest generation VNS device, the SenTiva Model 1000, and to the best of our knowledge, this study is likely the first to evaluate adverse effects associated with this model. Our study has limitations, including its retrospective nature and reliance on documentation from different providers who may employ different methods of recording information. The small sample size was also a limitation. The frequency of seizures, improvements, and severity of adverse effects were subjective and based on patient or caregiver reports, which may result in underreporting or omission of certain details. Furthermore, during the documented follow-up, changes were made to anti-seizure medications and VNS settings, making evaluation of the efficacy and side effects challenging.
Conclusion
Our study demonstrated that VNS serves as an important palliative adjunct treatment option for patients with medically refractory epilepsy. Specifically, this study examined the variables associated with adverse effects. Notably, dysphagia and dyspnea emerged as significant adverse effects, which may result in treatment discontinuation, aspiration pneumonia, ICU admission, and a complicated hospital stay. These effects are more prevalent among patients with symptomatic generalized epilepsy, GDD at baseline, previous ICU admissions, abnormal brain MRI findings, and seizures with multiple semiologies. When considering surgery, it is essential to take into account these patient characteristics during counseling, as well as to promote early evaluation for adverse events and prompt intervention following the procedure.
Acknowledgement
We would like to thank American Manuscript Editors for the English language editing.
- Received January 11, 2023.
- Accepted September 18, 2023.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.