Abstract
Objective: To investigate the effects of thymoquinone (TQ) in a penicillin-induced epilepsy model in rats.
Methods: This experimental study included 56 adult male Wistar rats. Experiments were performed in the Research Laboratory of the Department of Physiology, Medical School, Duzce University, Duzce, Turkey, between October 2013 and December 2014. Animals were divided into the following 7 groups: sham, control, only thymoquinone, vehicle (Dimethylsulfoxide), and doses of 10, 50, and 100 mg/kg of TQ. After rats were anesthetized, the left part of the skull was removed. A pair of silver/silver chloride electrodes was placed on the somatomotor area, and electrocorticographic recording was started. After 5 minutes basal activity was recorded, and TQ was applied intraperitoneally. At the thirtieth minute after TQ, epileptiform activity was induced by intracortical penicillin. The first spike latency, spike frequency, and the amplitude of epileptiform activity were analyzed statistically.
Results: The different doses of TQ significantly increased the latency time to onset of first spike wave, and decreased the frequency, and amplitude of epileptiform activity in the first 20 minutes compared with the control group.
Conclusion: Thymoquinone shows potential as an antiepileptic drug resulting from its effects of prolonged latency time, and reduced spike wave frequency and amplitude of epileptiform activity.
Epilepsy, characterized by recurrent spontaneous seizures, is one of the most common neurological disorders. Epilepsy is not only a disease, but also a symptomatic condition caused by genetic factors, traumatic brain injury, CNS infections, stroke, or structural brain lesions including brain tumors. Despite investigations, no cause can be found for the underlying etiology in approximately 65% of patients.1 At the present time, there are nearly 50 million people who have active epilepsy with frequent seizures requiring therapy. Approximately 30% of these patients are resistant to all antiepileptic medications discovered to date.2 Black cumin (Nigella sativa) has been used as a traditional remedy for many diseases for over 2000 years. The compounds in black cumin were isolated by pharmacological active solid-phase extraction and high performance liquid chromatography separation methods.3 The oil extracted from the seeds contains saturated (30-36% w/w) and unsaturated fatty acids, and volatile oil (1.1–1.4% w/w), major components of which are thymoquinone (TQ), dithymoquinone, and nigellone (polythymoquinone).4 Thymoquinone (C10H10O2; 2-isopropyl-5-methyl-1,4-benzoquinone; molecular weight 164.2) composes 27.8-57% of the volatile fatty acids of black cumin.3,4 In previous studies, many effects of TQ including antioxidant,5 anti-inflammatory,6 analgesic,7 anti-asthmatic,8 and antimicrobial9 effects were found, and a cell reproduction inhibitory effect in many types of cancer was also detected.10,11 It has very few documented side effects.12 In recent years, studies13 have suggested that TQ has a neuroprotective effect on the CNS. In these studies, TQ was demonstrated to inhibit apoptosis and neuronal degeneration in the cerebral cortex.13 Also, Nigella sativa oil and its active ingredient TQ, protects brain tissue against radiation-induced nitrosative stress.14 The TQ has a protective potential in the rat hippocampus and cortical neurons against Abb1-42 and thus, it may be a promising agent for the treatment of Alzheimer’s disease.15 A limited number of studies also reported that TQ has shown antiepileptic effects. Akhondian et al16 reported that orally administered TQ reduces intractable pediatric seizures. Also, Hosseinzadeh et al17 showed that TQ administered intracerebroventricularly, for epileptiform activity induced by using pentylenetetrazole (PTZ) in rats, prolonged the latency to first seizure, and decreased seizure count and the periods of tonic-clonic seizure in a dose dependent manner. In another study, orally administered TQ prolonged the first seizure latency, decreased seizure count, and eliminated lethality in PTZ-induced epilepsy.18 Hosseinzadeh and Parvadeh19 suggested that TQ activity increases GABAergic conduction via opioid receptors in a petit mal epilepsy model in mice. The anticonvulsant activity of the volatile oil of Nigella sativa seeds was attributed mainly (70%) to TQ, then to p-cymene (24%), and a-pinene (6%) in a maximal electroshock (MES) induced model. However, the TQ and other compounds did not exert any anticonvulsant effect, except p-cymene.20
The exact role of TQ, in the treatment of epilepsy is not known. Black cumin (Nigella sativa) is still being used as a traditional remedy for many diseases including epilepsy without an understanding of the scientific evidence. To be accepted as a therapeutic drug for epilepsy, the benefits of a substance must be shown in many animal models and species. Penicillin-induced experimental epilepsy in mice, rats, and cats is the prototype of focal motor seizures in humans. Providing electrophysiological evidence is the most important advantage of this model and thus, it is used frequently in epilepsy studies.21 The purpose of the present study is to investigate the effects of intraperitoneally administered TQ on penicillin-induced epileptiform activity via electrocorticogram in anesthetized rats.
Methods
Animals
Male Wistar rats (230-260 g, aged 12 weeks) were obtained from the Abant İzzet Baysal University, Experimental Animals Research Center, Bolu, Turkey, and housed in groups of 6 under standard laboratory conditions. They were kept at constant room temperature (21±2°C) under a 12/12 h light/dark cycle. Rats were given ad libitum access to food and water. Experiments were performed between 08:00-12:00 a.m. in the daylight period to avoid the effects of circadian variation. All animal experiments were carried out in accordance with the ethical guidelines of the Ethics Committee of the Abant İzzet Baysal University, and the NIH Guiding Principles in the Care and Use of Animals. The experiments were performed in the Research Laboratory of the Department of Physiology, Medical School, Duzce University, Duzce, Turkey, between October 2013 and December 2014.
Drugs and doses
Thymoquinone (Sigma-Aldrich Chemical Co., St. Louis, Missouri, USA) was administered intraperitoneally (i.p.) in 10 mg/kg, 50 mg/kg, and 100 mg/kg doses. Rapamycin was dissolved in Dimethylsulfoxide (DMSO, Loba Chemie, Mumbai, India) following dilution with saline (99% DMSO; 0.2 ml final solution DMSO/saline 1:4, v/v). Urethane (Sigma-Aldrich Chemical Co., St. Louis, Missouri, USA) in 1.25 g/kg i.p. doses was used as an anesthetic. Epileptic activity was stimulated by injecting 500 IU/2 µl intracortical penicillin into somatomotor cortex with a Hamilton microinjection (701N, Hamilton Co., Reno, NV, USA). The injection coordinates are 2 mm lateral, 1 mm anterior, and 1.2 mm depth of Bregma line.
Surgical procedure
The surgical, electrophysiological recording, and obtaining of data methods is the same as described in previous publications.21-23 Each of the animals in all groups was anesthetized with urethane, and fixed onto a stereotaxic frame (Harvard Instruments, South Natick, MA, USA). After shaving the head area, the scalp was incised through the midline, from front to back with a scalpel. The bone above the left cerebral cortex was slenderized with a drill (Proxxon Minimot 40/E, Proxxon GmbH, Niers-bach, Germany), and carefully removed.
Experimental groups
The animals were divided into groups as follows: Group 1 - Sham group, which only underwent surgical procedures [n=8]. Group 2 - Only TQ group (100 mg/kg i.p.) [n=8]. Group 3 - Solvent (vehicle) group, which received DMSO (1.1 mg/kg or 1 ml/kg i.p.) [n=8]. Group 4 - Control group, which only received Penicillin G (500IU/1µl, i.c.) [n=8]. Group 5 -10 mg/kg TQ group, which received TQ + Penicillin G [n=8]. Group 6 - 50 mg/kg TQ group, which received TQ + Penicillin G [n=8]. Group 7 - 100 mg/kg TQ group, which received TQ + Penicillin G [n=8].
Electrophysiological records
Two silver/silver chloride (Ag-AgCl) top electrodes were placed in the somatomotor cortex area, which was opened on the left hemisphere lateral to the Bregma line. After the electrodes were placed, electrocorticography (ECoG) recordings (PowerLab/8SP, ADInstruments Pty Ltd, Castle Hill, NSW, Australia) were taken throughout the experiment. Before application of TQ, a 5 minute basal activity recording was taken. Thereafter, at the thirtieth minute of TQ application, epileptiform activity was induced by intracortical administration of penicillin (500 IU). Analysis of the obtained records was performed using the PowerLab Chart v.6.0 software package (ADInstruments Pty Ltd., Colorado Springs, CO, USA). Epileptiform activity occurring in bipolar spike and spike wave complexes was examined. Additionally, the mean, median, minimum, and maximum, quartile 1 (Q1) and quartile 3 (Q3) values of spike wave frequency and amplitudes per minute in the 5 minute-periods of ECoG recordings of each animal were measured and used as data.
Statistical analysis
The latency time to onset of first spike wave, spike wave frequency, and amplitude data were digitized and computed from the records of each animal using Chart software (ADInstruments Pty Ltd., Colorado Springs, CO, USA). Kruskal-Wallis test was used for measuring differences between groups in latency, frequency, and amplitude data. Dunn test, followed by Kruskal-Wallis test, were used to determine statistically significant differences between groups. Type I error was accepted as 0.05, and the PASW software (Predictive Analytics SoftWare Statistics version 18.0 (SPSS Inc Ltd, Hong Kong, Republic of China) was used for all statistical computations.
Results
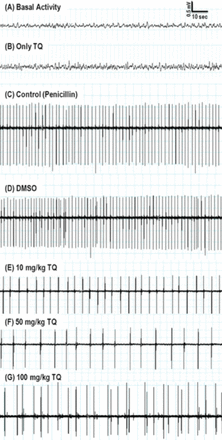
There was no effect of the applied substances on the basal activity that was recorded before penicillin administration (Figure 1). Both the TQ and DMSO applied after the 5 minute basal activity recording did not cause any epileptiform activity in the 30 minutes ECoG recordings, which were also taken before penicillin administration. Moreover, no epileptiform activity was observed during recordings in the TQ only administered group, and the sham group.
Representative electrocorticography (ECoG) samples are presented in the 5-10 minutes from chemicals, or in the 15-30 minutes from penicillin administration. A) Baseline ECoG activity before penicillin injection. B) Epileptiform activity was not observed during recordings in the thymoquinone (TQ) only administration group. C) Intraperitoneal injection of Dimethylsulfoxide (DMSO) did not change the frequency of penicillin-induced epileptiform activity. D) Intracortical injection of penicillin (500 IU) induced epileptiform activity on ECoG. E, F, & G) Administration of TQ (10, 50, and 100mg/kg i.p.) decreased the frequency of epileptiform activity.
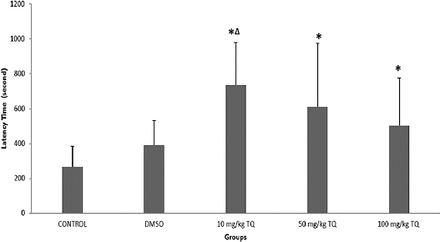
Latency of the first epileptiform activity
After penicillin injection, spike waves of epileptiform activity were first seen between 5 and 10 minutes (Figure 2). The median latency value of the control group was 266, with a value of 390 for the DMSO, 735 for the 10 mg/kg TQ, 610 for the 50 mg/kg TQ, and 502 for the 100 mg/kg TQ groups. When the groups were compared in terms of latency of the first epileptiform activity, there was a statistically significant difference (p=0.015). When examined in detail, the latency values of the TQ groups were significantly longer than the control group. In addition, the latency of the 10 mg/kg TQ group was longer than the 50 mg/kg TQ, and the 100 mg/kg TQ groups, but this difference was not statistically significant. There was a statistically significant difference between the 10 mg/kg TQ group and the DMSO group (p=0.018) (Figure 2).
Latency of the first epileptiform activity. *Significance compared with the control group (p<0.05); ∆significance compared with the dimethylsulfoxide (DMSO) group (p<0.05).
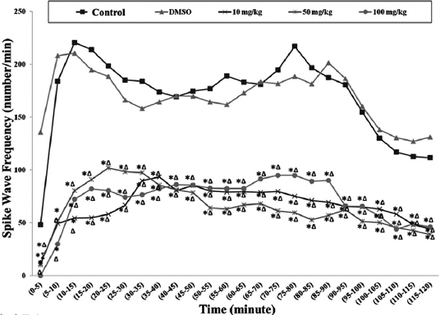
The effects of thymoquinone on spike wave frequency
Following the penicillin administration, spike wave frequencies of epileptiform activity in the first 5 minutes were significantly lower in the TQ group, compared with the control and DMSO groups. The median values of spike wave number per minute were significantly lower in course of time in each of the 3 TQ dose groups (Figure 3).
Median values of spike wave frequency (number/min) obtained from recording after penicillin. *Significance compared with the control group (p<0.05); ∆significance compared with the dimethylsulfoxide (DMSO) group; ∑significance compared with the 50 mg/kg thymoquinone (TQ) group (p<0.05).
The spike wave frequency median values were found to be; 144/min in 40th minutes and 98/min in 105th minutes in control group, and 178/min 45th minutes and 138.50/min in 100th minutes in DMSO group. The spike wave frequency median values in the 10, 50, and 100 mg/kg TQ groups between 40th and 45th minutes were 76, 74, and 85 per minute. The spike wave frequency median values at the same doses between 105th and 110th minutes were 39.50 for 10 mg/kg group TQ, 12.00 for 50 mg/kg TQ group, and 30.50 100 mg/kg TQ group per minute.
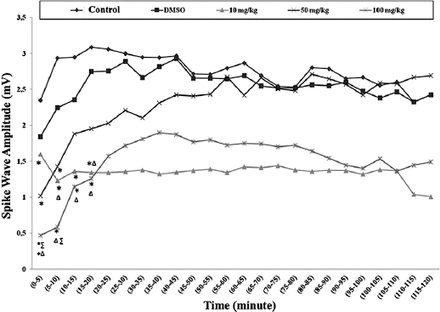
The effects of thymoquinone on spike wave amplitude
There were generally significant decreases in the spike wave amplitude medians in each of the 3 TQ dose groups compared with the control group in all time periods between 0 and 20 minutes. When they were compared with the DMSO group in the same time periods, there was a significant decrease in only the 100 mg/kg TQ group (Figure 4). There was no significant difference among groups between 20 and 120 minutes, in terms of amplitude (p>0.05).
Spike wave amplitude (mV) median values obtained from recording after penicillin. *Significance compared with the control group (p<0.05); ∆significance compared with the dimethylsulfoxide (DMSO) group; +significance compared with the 10mg/kg thymoquinone (TQ) group (p<0.05); ∑significance compared with the 50 mg/kg TQ group (p<0.05).
Discussion
In the present study, the effects of TQ were investigated when administered intraperitoneally at 10, 50, and at 100 mg/kg doses on penicillin-induced epileptiform activity in anesthetized rats. The TQ administration without penicillin-induced epilepsy, and the DMSO that was used as a vehicle, did not cause epileptiform activity in any of the animals. The data shows that administering TQ and DMSO to non-epileptic rats did not cause epileptic effects.
In the ECoG recordings obtained from the animals with epileptiform activity induced by penicillin, the TQ, which was administered 30 minutes before penicillin, prolonged the epileptiform activity latency in the 10, 50, and 100 mg/kg dose groups when compared with the control and DMSO groups. The DMSO made an insignificant small extension in latency, compared with the control group. The obtained results are consistent with the latency results obtained in PTZ and MES epilepsy models.17
Different doses of TQ decreased epileptiform activity spike wave frequency in all the 2-hour time periods except for the first 5 minutes, when compared with the control and DMSO groups. The difference in the first 5 minutes may have originated from both the irregularity of the epileptiform activities in the beginning, and the fact that DMSO slightly increases the activity frequency in the first 5 minutes. As it was seen, TQ has protective effects on epileptiform activity throughout 150 minutes. This finding may be important since the electrophysiological effect of TQ on epilepsy is not well reported in the literature. The fact that all the TQ doses significantly decreased spike wave amplitude in the first 20-minute time period was shown. The electrophysiological effects lasted around 50 minutes in total, and this finding is a notable contribution to the literature.
Inhibitor pathways that act on the gamma-aminobutyric acid (GABA) receptors that are ion channels, are accepted as the most important suppressor control system in the brain. Decreasing or eliminating this inhibitor activity is thought to cause epileptic discharges.20 Decreased inhibitor activity impairs the inhibitory-excitatory balance towards excitatory glutamate, and causes the formation of suitable conditions for the onset and propagation of epileptic activity.24 Sometimes, over-activation of a glutamate receptor type, N-methyl-D aspartate (NMDA), may also cause epileptiform activities.25 In both conditions, the resulting epileptiform activity is decreased with competitive or non-competitive NMDA channel antagonists.22
In the CNS, GABA (GABAA and GABAB) receptors are suppressed when they are exposed to picrotoxin or the competitive antagonist bicuculline, and cause epileptiform activity, and this activity may be prevented with various antagonists. The GABA binding to GABA receptors leads to the influx of the chloride ions (Cl-) through selective Cl channels into the cells and hyperpolarization or inhibition of the cells occurs.20 Potentials linked to GABAA and GABAB receptors, contribute to the fast depolarization shifts observed in cortical pyramidal cells in a penicillin-induced epilepsy model.26 Researchers have speculated that picrotoxin-sensitive GABA receptors and bicuculline-sensitive GABAA receptors are involved in the anticonvulsant activity of thymoquinone.20 Penicillin directly administered into the cortex, shows an effect similar to bicuculline after 5-10 minutes, and causes inhibition of GABA receptors, and thus the suppressed GABA activity interrupts the inhibitory system of the brain, and starts local epileptiform activity.23
Studies that have been performed to determine the effects of TQ on CNS, suggest an involvement of nitric oxide-cyclic guanosine monophosphate (NO-cGMP) and GABAergic pathways in the anxiolytic-like activity of thymoquinone.27 Inhibition of brain oxidative stress and inducible NO synthase expression by thymoquinone attenuates the development of morphine tolerance and dependence in mice.28 These studies suggest that the TQ may show its effect via NO. Nitric oxide was studied as a gas neurotransmitter in epileptogenesis and found to be a proconvulsant.23 Another proposed mechanism of TQ is through its action on depression of intracellular calcium release from its stores by NO.17
It was reported that the TQ can increase GABAergic transmission via opioid receptors in a petit mal epilepsy model in mice.19 As it is known that the opioid kappa receptor agonists usually affect Ca2+ channels and blocks cellular Ca2+ influx,29,30 these results suggest that the anticonvulsant effect of TQ may also be conducted via opioid kappa receptors. The TQ may also show antiepileptic effects by decreasing cellular Ca2+ influx and/or inducing GABA due to its co-localization with GABA.
Our findings, which show that TQ prolongs epileptiform latency activity, are consistent with the literature. The effects of TQ on spike wave frequency and amplitude have not been previously reported in the literature.17-20 Hosseinzadeh et al17 demonstrated that intraperitoneal administration of TQ in 20, 40, and 80 mg/kg doses decreased the epileptiform activity in a PTZ-induced epilepsy model. These investigators reported that administering 40 mg/kg dose of TQ 30 minutes before PTZ administration prolonged the latency of the first seizure from 44.4 seconds to 128.5 seconds, and decreased the total seizure period from 12.2 seconds to 9.9 seconds. In addition, they reported that administering a 40 mg/kg dose of TQ 60 minutes before PTZ administration, prolonged the latency of the first seizure from 44.4 seconds to 265.7 seconds, and decreases the total seizure period from 12.2 seconds to 5.8 seconds. According to these studies, the optimum time for the anticonvulsant activity of TQ appears to be the first 60 minutes after its administration.19 This may be related to the slow transmission of TQ from the peritoneum to the circulation, and then to the blood-brain barrier. In our study, administration of TQ, 30 minutes before penicillin, prolonged the latency of the first seizure and these latencies in all of the groups were lower than other studies. This difference may be caused by the fact that our study is limited to electrophysiological observations, and does not evaluate motor seizures.
Thymoquinone was also studied in other epilepsy models. It has been shown that TQ potentiate the antiepileptic effect of valproate in maximal electroshock induced epilepsy models in mice. However, this effect was less than PTZ model.20 It is also effective in kindling seizure models. However, some epileptic medications including flumazenil and naloxone decrease the anticonvulsant effects of TQ.17 In the present study, it was also observed that administration of TQ before penicillin prolongs the latency period and decreases the spike wave frequency in all 3 doses. This aspect of the study suggests that the TQ has an anticonvulsant effect against epilepsy.
The route of administration of substances, models, and animal species used in the experiments may give different results. Thymoquinone administered by i.p., oral, and intracerebroventricular routes in epilepsy models such as PTZ and MES in rats and mice, was reported to prolong the latency of the first seizure, decrease the seizure frequency, and eliminate lethality.17-20 Akhondian et al1 investigated the antiepileptic effect of orally administered TQ on 22 pediatric patients with antiepileptic drug resistant epilepsy. They reported that the seizure frequency of the TQ group was significantly lower than the placebo group. The antiepileptic effects noted with orally and intracerebroventricularly administered TQ in previous studies were also observed with i.p. administered TQ in our study.
Nigella sativa has many ingredients including volatile and non-volatile oils. The TQ used in our study constitutes the majority of volatile oils. To date, Nigella sativa seed ingredients were tested as a whole or separately in the studies utilizing epilepsy models. In a study performed in mice, the Nigella sativa oil tested was found to be effective against the PTZ model.18
Dimethylsulfoxide is frequently used as a solvent in studies performed with antiepileptic substances.31-33 Researchers reported that DMSO decreases seizure threshold, and augments the proconvulsant activity of the substances dissolved in DMSO.33 Intraperitoneal DMSO administration altered the absence-like epileptic seizure activities in freely moving WAG-Rij rats.32 The DMSO was reported to have a dual effect since it decreased the spike wave frequency at low doses (1.65mg/kg or 1.5 ml/kg), and contrary to this, it increased the spike wave frequency at high doses (1650.6 mg/kg). In our study, the DMSO had no effect on latency of the first epileptiform activity, the spike wave frequency, and spike wave amplitude. The fact that our dose is much lower than other studies probably precluded this effect, and the DMSO had no effect on the experiment as desired.
In conclusion, we demonstrated that TQ has a protective and inhibitory effect on a penicillin epilepsy model, as with the other experimental epilepsy models. We did not perform molecular and biochemical analyses in this study, but only investigated the effect on epileptiform activity electrophysiologically. This is the major limitation of the study. Conducting multidisciplinary studies involving biochemical and histological studies will help to provide more information on the biological effects of TQ. Further research on the underlying mechanism of TQ in epilepsy is required before it gains widespread use in clinical practice.
Footnotes
Disclosure
This study was supported by the Committee for Scientific Research of Düzce University. The authors declare no conflicting interests, support or funding from any drug company.
- Received December 14, 2015.
- Accepted January 20, 2016.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.