Abstract
Burr-hole craniostomy with closed-system drainage is a safe and effective method for the management of chronic subdural hematoma. However, contralateral acute subdural hematoma has been reported to be a rare and devastating complication. Only 3 cases have been described in the literature. Herein, we reported an 80-year-old male with chronic subdural hematoma and contralateral subdural hygroma. The burr-hole craniostomy with closed-system drainage was initially performed to treat the chronic subdural hematoma. Three days after surgery, weakness of the extremities developed, and contralateral acute subdural bleeding within the previous subdural hygroma was diagnosed by CT scan of the brain. The pathophysiological mechanism of this rare complication was discussed, and the relevant literature was also reviewed.
Chronic subdural hematoma (SDH) is one of the most common entities managed in daily neurosurgical practice, and is known to have a good prognosis after minimal burr-hole craniostomy.1 Ipsilateral acute SDH or recurrence of hematoma is the most well-documented complication of this simple surgery.1-3 However, the development of a contralateral acute SDH following burr-hole craniostomy with closed-system drainage has been previously reported to be a rare but devastating postoperative complication.4,5 Herein, we report a case of contralateral acute SDH occurring after evacuation of chronic SDH with the coexistence of contralateral subdural hygroma. The relevant literature is also reviewed. Our objective in presenting this particular case is to highlight this rare but devastating complication in the management of this disease.
Case Report
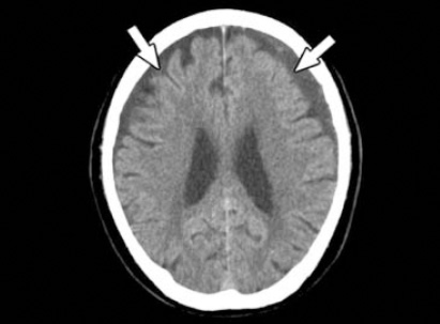
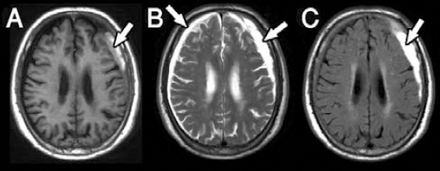
An 80-year-old male was admitted to our hospital with a history of head injury resulting from a fall 2 months previously. He had complained of dizziness, right side motor weakness, and unsteady gait one week prior to admission. His medical history revealed no chronic disease or use of any anticoagulant agents. Neurological examinations revealed muscle strength in the right side extremities scored as grade 4 over 5 on the Medical Research Council Scale. The laboratory examinations were within normal limits and no bleeding diathesis was noted. A CT scan of the brain revealed hypodensity lesions in the bilateral frontotemporoparietal region that were larger on the left side (Figure 1). To exclude acute stroke, a brain MRI was performed. The MRI revealed a crescentic accumulation of subdural fluids with hyperintensity on T1- and T2-weighted images on the left side, and hypointensity on T1-weighted, and hyperintensity in T2-weighted images on the right side (Figures 2A & 2B). High signal intensity in the left side fluid was revealed in the sequence of fluid-attenuated inversion recovery images (Figure 2C). A subdural hygroma on the right side, and chronic SDH on the left side were diagnosed.
Preoperative brain CT demonstrating bilateral subdural effusions (arrows).
An MRI of the brain demonstrating the subdural hygroma on the right side and chronic SDH on the left side. A) T1-weighted images revealed low signal intensity on the right side and high signal intensity on the left side (arrow). B) T2-weighted images revealed high signal intensities on both sides. C) Fluid attenuation inverse recovery sequence revealed low signal intensity on the right side and high signal intensity on the left side (arrow).
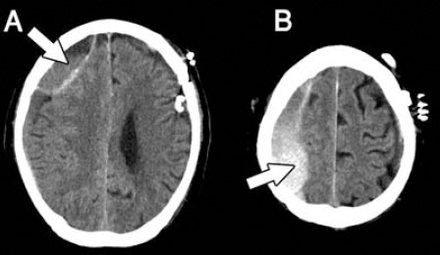
After explanation, he underwent a burr-hole craniostomy with closed-system drainage for the left side chronic SDH. Under general anesthesia, the lesion was evacuated through one burr-hole. Moderate pressure of the motor-oil-like fluid was noted during dural opening. Irrigation with normal saline in the subdural space was performed, and then a closed drainage system was placed. No active bleeding or rapid expansion of the brain parenchyma was observed during the procedure. However, weakness in the left side extremities developed 3 days after surgery. Muscle strength in the left side extremities was scored as grade 3 over 5. The subsequent CT revealed the development of acute hemorrhage within the previous subdural hygroma space in the right side frontal and parietal area, compressing the adjacent parenchyma (Figure 3). Then, emergency right side burr-hole craniostomy with closed-system drainage was performed. High intracranial pressure was noted during dural opening and no obvious blood clot formation was found. The symptoms improved gradually within 2 weeks postoperatively.
Postoperative brain CT demonstrating acute subdural hemorrhage within the hygroma space in the right frontal A) and parietal B) regions (arrows).
Discussion
Chronic SDH forms from progressive ongoing bleeding around the outer and inner membranes. Most chronic SDHs occur in elderly patients, with a male preponderance.2 Brain atrophy, a history of head trauma, the presence of acute SDH, or concomitant coagulopathy has been described as positive risk factors.1,2 Excessive activation of both coagulation and fibrinolytic systems, or a high expression of tissue-type plasminogen activator in hematomas has been proposed as a possible explanation for the failure of hematoma to coagulate.6 The management of chronic SDH is approached with various techniques, including twist-drill craniostomy with catheter drainage, burr-hole craniostomy with closed-system drainage, endoscopic removal of hematoma, subdural-peritoneal shunt, or large craniotomy with removal of hematoma and membranectomy.2 A positive functional outcome has been achieved in 72-93% of patients, depending on the treatment method.1 However, burr-hole craniostomy with or without drainage is the most frequently used technique and is considered as a minimal and effective method to treat chronic SDH.3,7
Although the overall rate of postoperative complications has not been clearly reported, the most common complications in patients with chronic SDH managed by burr-hole craniostomy include recurrence, acute ipsilateral sub- or epidural hematoma, epilepsy, intracranial hypotension, subdural empyema, intracerebral hemorrhage, and tension pneumocephalus.1-3,8 Postoperative contralateral acute SDH after burr-hole craniostomy has been reported to be a rare but devastating complication.1,2,8 Only 3 case reports, including one huge bilateral chronic SDH,5 one subdural hygroma,9 and one unilateral chronic SDH10 were found in the literature. Acutely deteriorated symptoms were found in all these patients within several hours after the initial burr-hole craniostomy, and the subsequent CT scan of the brain confirmed the diagnosis of contralateral acute SDH. Although the acute hematoma may become clinically apparent within hours or days after minimally invasive trephination surgery, to the best of our knowledge, there have been no case reports describing the development of contralateral acute SDH within the previous hygroma after initial burr-hole craniotomy for chronic SDH in which the clinically deteriorated symptoms were observed 3 days postoperatively.
The pathophysiological mechanism of developed contralateral acute hematomas after evacuation of chronic SDH is considered to be a dynamic and not a static entity.2 The high intracranial pressure causes the subdural fluid to be ejected from the dural opening, which leads to brain shift that can tear the bridging veins and result in contralateral SDH.11 Otherwise, the intraoperative dehydration and aggressive CSF aspiration may contribute to the perioperative brain parenchymal shift.9 It has also been hypothesized that the sudden onset of a restoration of normal perfusion pressure in areas of faulty cerebral vascular autoregulation may lead to vascular damage that results in acute bleeding.12 Therefore, slow decompression with closed-system drainage is considered to avoid rapid dynamic intracranial changes, including brain shift or restoration of normal perfusion during decompression of the brain, and prevent these devastating complications.13 However, when contralateral acute hematoma develops after initial burr-hole craniostomy, the treatment strategy depends on the size of the hematomas, the presence of a midline shift, and the neurological function of the patients. With a prompt CT scan of the brain to assess this rare complication, the adverse outcome may be avoided.9
In our case, the chronic SDH and contralateral subdural hygroma were confirmed by the MRI study. The burr-hole surgery with closed-system drainage was initially performed to treat the chronic SDH. However, weakness of the limbs developed 3 days postoperatively. The subsequent CT scan of the brain revealed acute SDH within the contralateral subdural hygroma, compressing the brain parenchyma. The clinical symptoms improved gradually after emergent right side burr-hole craniostomy with closed-system drainage. This rare complication may have resulted from the brain shift with tearing of the contralateral bridging veins during drainage of the ipsilateral chronic SDH. Otherwise, in a review of 106 patients with 126 chronic SDHs, the subdural hygroma was found to have changed into a homogenous hematoma after an average of 28 days.14 The chronic SDH may have originated from the initial subdural hygroma. Therefore, in our case, the previous subdural hygroma may have been the predisposing factor activating the acute course of SDH. That may be why the acute bleeding within the previous hygroma space after burr-hole craniostomy with closed-system drainage and no obvious blood clot formation was found intraoperatively.
In conclusion, although burr-hole craniostomy with closed-system drainage is an effective method to treat chronic SDH, contralateral acute SDH may occur and become a devastating complication, especially with the coexistence of contralateral subdural hygroma.
- Received October 28, 2013.
- Accepted March 18, 2014.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.