Abstract
Tardive dyskinesia (TD) is one of the most serious and disturbing side-effects of dopamine receptor antagonists. It affects 20-50% of patients on long-term antipsychotic therapy. The pathophysiology of TD remains poorly understood, and treatment is often challenging. Here, we present a 32-year-old woman presenting with a 9-month history of TD occurring after risperidone withdrawal, and characterized almost exclusively by tongue protrusion. After being seen by different specialties and undergoing multiple investigations, she was eventually correctly diagnosed with TD by a specialist team and successfully treated with amantadine. Vigilance and awareness of this condition and its risk factors are required to make the correct diagnosis, especially in cases with unusual presentations caused by atypical antipsychotics, and treatment can be challenging.
Tardive dyskinesia (TD) describes a complex of abnormal movements involving almost any muscle in the body (most commonly the oro-facial, buccal, and lingual muscles) in patients on long-term dopaminergic antagonist mediations.1,2 It can present with a range of abnormal movements including akathisia, dystonia, tics, tremor, chorea, or their combination.2 Although classically stereotypic in nature, TD can present with any muscle abnormality,2 which can lead to diagnostic difficulties. Tardive dyskinesia is particularly debilitating for the patient when it affects swallowing, speaking, loss of skilled movements, or pain, and this in turn can lead to social withdrawal and poor drug compliance; TD therefore has biological, psychological, and social impacts.3 Although atypical antipsychotics are less likely to cause TD than typical antipsychotics,2 we present a case of TD occurring after risperidone withdrawal in a young woman presenting almost exclusively with tongue protrusion. Our objective in presenting this particular case is to highlight challenges in diagnosing TD with its variable and unusual presentation and the level of evidence of different medications.
Case Report
A 32-year-old single female with insulin-dependent diabetes mellitus and hypothyroidism was referred from a primary care with a protruded tongue, and difficulty in speech and swallowing after reducing her dose of risperidone. Her signs worsened after medication was discontinued.
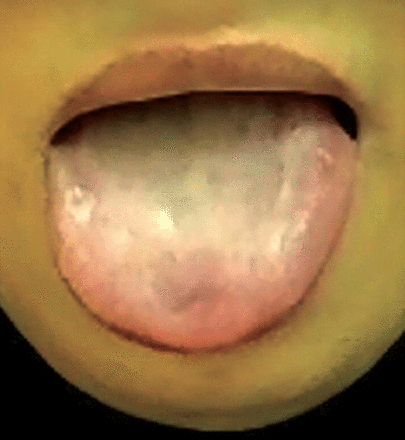
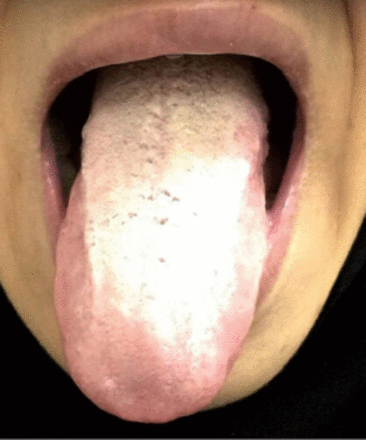
Two years previously, she had experienced a psychotic episode without mood symptoms. She was diagnosed with brief psychotic disorder, and was treated with oral risperidone 2 mg nightly. The clinical response was excellent, and she settled to baseline; as a result, after one year, the risperidone dose was decreased to one mg. At this point, her tongue became enlarged and protruded, and she developed swallowing difficulties, nasal speech, and hypersalivation that worsened after discontinuation of risperidone. Outpatient medical advice was sought in several secondary and tertiary urban hospitals. She was eventually admitted to a specialist neurology team for 2 weeks of thorough neurological work-up. On admission, she was found to have nasal speech, difficulty swallowing, hypersalivation, a swollen and protruded tongue, and weak muscles of mastication. There was no eye droop, double vision, symptom fluctuation, diurnal variation, or fatigability. The provisional diagnosis was myasthenia gravis, and she was started on oral prednisolone 10 mg daily and oral pyridostigmine bromide 60 mg twice daily with no improvement in signs or symptoms. Her full blood count, urea and electrolytes, and liver and renal function tests were within normal limits, glucose and glycosylated hemoglobin (HbA1c) were raised at 11.2 mmol/L (normal ranges: 4.0 to 5.9 mmol/L) and 11.30% (normal range: 4-6%) and thyroid function tests were abnormal with a TSH of 14.68 mIU/L (raised) (normal ranges: 0.5 and 4.0 mIU/L), but a free T4 of 15.740 pM/L (normal). Acetylcholine receptor antibody titers were negative. She was intolerant to repetitive nerve stimulation, and a CT of the head, neck, and chest was normal. She was discharged with a diagnosis of a “motor disorder not otherwise specified,” with instructions to undergo further investigations. All medications were discontinued, and a referral to otolaryngology and a swallowing team revealed no abnormalities. She was subsequently seen in an outpatient specialist psychosomatic medicine clinic. She exhibited no positive or negative psychotic features, cognitive impairment, or manic, hypomanic, or depressive features. She was visibly distressed by the difficulties she was experiencing in talking and swallowing, and she was concerned regarding the diagnosis and management of her condition. However, she did not have a diagnosable anxiety disorder, and there was no suicidal or homicidal ideation. A diagnosis of risperidone withdrawal-induced TD was made. The diagnosis presented a number of challenges to the psychiatry team: she was visibly suffering, her family was exhausted from multiple admissions and hospital appointments in different cities, there was erosion of trust in physicians, and there was a growing sense of hopelessness and helplessness due to the disturbing diagnosis and known difficulties in treating TD. Treatment was commenced with a full explanation of the diagnosis, known challenges to therapy, and expected outcomes to both the patient and her family. Figure 1 shows patient tongue on her initial presentation to clinic. Haloperidol 0.5 mg was started on the basis of weak evidence that full dopamine receptor antagonism can be beneficial in TD, with follow-up after one week. However, she discontinued haloperidol after 2 days due to extrapyramidal side effects. Due to local availability, 2 other treatment options were available: clonazepam (level B evidence) and amantadine (level C evidence). Since she wanted to avoid medications that carried a risk of addiction, 50 mg oral amantadine once daily was started, to be increased after 4 days to 100 mg daily with follow-up after 2 weeks. The patient responded well (50% improvement), and the dose was increased to 100 mg twice daily with 80% improvement. She quickly returned to her social life, and she remained satisfied after 4 months of amantadine therapy. Although an increased dose of 300 mg daily was offered, the patient refused since she felt better and continued to improve. She remains well on amantadine at follow-up, and the intention is to discontinue therapy in time (Figure 2).
An image showing the patient’s tongue at initial presentation at a clinic in Riyadh, Saudi Arabia.
An image showing the patient’s tongue after 6 months of therapy.
Discussion
Tardive dyskinesia is one of the most disturbing side effects of antipsychotic medication, and it is potentially irreversible.1 It describes a hyperkinetic movement disorder of delayed onset that usually occurs after prolonged use of dopamine receptor antagonists, particularly antipsychotic and antiemetic drugs.2 Tardive dyskinesia was first named and classified in 1964,2 and it is estimated to occur in approximately 20-50% of people receiving long-term neuroleptic therapy.2 According to the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5),4 TD is defined as “involuntary athetoid or choreiform movements (lasting at least a few weeks) generally of the tongue, lower face and jaw, and extremities (but sometimes involving the pharyngeal, diaphragmatic, or trunk muscles) developing in association with the use of a neuroleptic medication for at least a few months.” However, the most recent definition of TD is broader and includes tardive dystonia as a TD syndrome subtype.2,9 It is very uncommon for TD to present only with tongue protrusion (as in our case),5 although tongue protrusion is recognized as a sign of TD.6 Symptoms of TD may develop after a short period of antipsychotic medication use, particularly in older patients. It is known that the movements seen in TD can appear after a discontinuation, change, or reduction in the dose of neuroleptic medications: this is known as neuroleptic withdrawal-emergent dyskinesia. Since withdrawal-emergent dyskinesia is usually time-limited (less than 4-8 weeks), dyskinesia persisting beyond this window is considered to be true TD.7 The pathophysiology of TD is poorly understood, but it is postulated that the basal ganglia are hypersensitive in patients with TD since greater D2 receptor blockade at the trough levels of neuroleptics has been associated with increased severity.7 Although advanced age is a major risk factor for TD,8 TD occurs at all ages, including young patients: in one study, 5.9% of patients aged 7-21 years receiving dopamine antagonists for 3 months developed TD.9 The prevalence of TD is 29% in elderly patients receiving dopamine antagonist treatment for 3 months, and 26-67% in patients on long-term treatment. Elderly female patients appear to be particularly susceptible to the development of TD,8 while young men are more prone to developing tardive blepharospasm and tardive dystonia. Tardive dyskinesia has been described in all ethnic groups,8 with overall prevalence’s ranging from 1-65% and Africans and African Americans appearing to be especially vulnerable to TD after exposure to low doses of neuroleptics for short durations.
Diabetes mellitus, which was present in this case, is a risk factor for TD.2 The symptoms of TD usually first appear after 1-2 years of continuous exposure to dopamine receptor antagonists and almost never before 3 months.10 The incidence of TD is lower in patients treated with second-generation neuroleptics (risperidone, olanzapine, quetiapine, Amisulpride, and ziprasidone), with the total annual incidence ranging from 0.8% in patients younger than 50 years to 5.3% in those older than 50 years.2 Taken together, this data suggests that the development of TD was perhaps unlikely to occur in this case since the patient was young, and had only taken the second-generation antipsychotic for one year at a low dose. Although TD completely or partially remits in some patients a few years after discontinuation of the offending medication, or even while continuing treatment, it tends to persist for years or decades. In one study, 33% of patients experienced remission of their TD 2 years after discontinuation of the offending drug.2
According to evidence-based guidelines (Guideline Development Subcommittee of the American Academy of Neurology),11 a number of treatment options are available: i) clonazepam and ginkgo biloba probably improve TD (Level B); ii) amantadine and tetrabenazine may also be considered for TD (Level C); iii) however, data are insufficient to support or refute the use of a number of other agents, or by withdrawing causative agents or switching from typical to atypical dopamine receptor antagonists (Level U). Other treatments also showing promise are branched-chain amino acids,12 which have been used in 18 patients with good outcomes and without serious side effects, and dextromethorphan, which blocks the glutamate NMDA receptor and suppresses involuntary movements in Parkinson’s disease, which was effective in 3 TD patients.13 However, long-term assessment of new agents, especially with respect to potential side effects, such as abuse and hallucinations, is required.13 Furthermore, one randomized controlled trial reported significant improvements in TD with a 12-week treatment of levetiracetam (dose up to 3000 mg per day); however, trial dropout was high.14
Although dopamine depletion agents, especially tetrabenazine, are considered first-line therapies and are effective for persistent and disabling TD,2 its expense, side effect profile (depression, lethargy, and parkinsonism) and unavailability in our hospital precluded its use. In our case, the use of amantadine was supported by the results of a controlled trial investigating TD treatment,15 availability in our hospital, and the lack of addictive potential: it was, therefore, our drug of choice. However, the length of treatment with amantadine for TD remains unclear, since there is currently no data on optimal treatment length in the existing literature.14
In summary, TD always needs to be considered in patients of any age presenting with unusual movement, or neuromuscular disorders when there is a history of antipsychotic medication use. Further controlled studies are needed to develop and refine the guidelines for managing TD.
Footnotes
Disclosure
This study was supported by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University, Riyadh, Kingdom of Saudi Arabia. The authors declare no conflicting interests, support or funding from any drug company.
- Received January 29, 2015.
- Accepted May 25, 2015.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.