Abstract
Objectives: To study the clinical features of Guillain–Barré syndrome (GBS) and the relationship between serum cystatin C (cysC) levels and Hughes motor scale (HMS) in GBS.
Methods: One hundred and one GBS patients between January 2017 and January 2020 were reviewed retrospectively. Their epidemiological characteristics, clinical manifestations and auxiliary examinations were assessed. The HMS was used to measure the peak deficit. The influencing factors of GBS severity were analyzed by univariate and multivariate analyses.
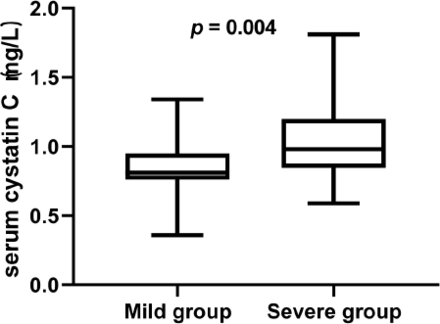
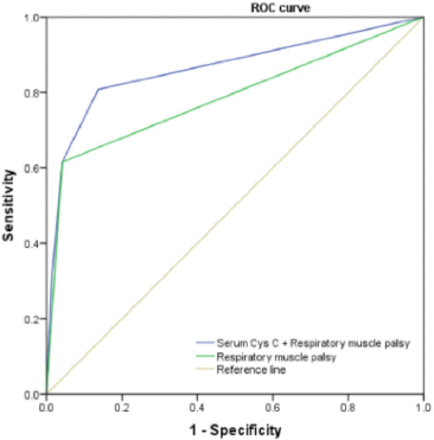
Results: The serum cysC levels were significantly higher in the severe group than in the mild group [0.98 (0.85-1.20) vs. 0.81 (0.76-0.95), p=0.004], and there was a positive correlation between serum cysC levels and HMS in GBS patients (r=0.376, p=0.001). On multivariate analysis, respiratory muscle palsy (p=0.003), time to peak deficit (p=0.017), serum cysC (p=0.045) and hyponatremia (p=0.015) were independent risk factors for a poor functional outcome (HMS>3). Combining serum cysC and respiratory muscle palsy was more valuable for assessing disease severity than respiratory muscle palsy alone (AUC 0.863 vs. 0.787, p=0.001).
Conclusion: Serum cysC was an independent risk factor in GBS, and positively correlated with HMS. It might be used to assess the severity of GBS with other negative prognostic factors.
Guillain–Barré syndrome (GBS) is an immune-mediated inflammatory disease of the peripheral nerves and nerve roots involving mainly the myelin sheath. Common methods for diagnosing GBS are neuro-electrophysiological and cerebrospinal fluid (CSF) examinations. In some atypical cases, however, these tests are not diagnostic in the early stage. Moreover, the molecular mechanisms underlying the disease remain poorly understood and no reliable disease markers are available. Cystatin C (cysC) is a cysteine protease inhibitor, that is constantly expressed in mammals; its level reflects changes in the glomerular filtration rate. Recent studies have found that some neurological diseases are associated with imbalances between cysC and cysteine proteases.1 A decrease in cysC has been reported in the CSF of GBS patients.2,3 Compared to lumbar puncture and electrophysiology examinations, serum cysC is simpler and easier to obtain. If serum cysC can provide some information for diagnosing and evaluating GBS, it would be useful.
In this study, we compared the serum cysC levels in GBS patients with mild and severe, and evaluated the risk factors that are associated with the severity of GBS to explore the role of cysC in GBS.
Methods
The clinical data of patients who were diagnosed with Guillain–Barré syndrome, Guillain–Barré syndrome axonal type, or Guillain–Barré syndrome demyelinating type in the Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China between January 2017 and January 2020 were reviewed retrospectively. One hundred one patients satisfied the clinical diagnostic criteria of GBS were enrolled.4 Patients with peripheral neuropathy, such as diabetic, vasculitis, toxin, hypokalemia, rabies, porphyria and drug-induced diseases were excluded, as were patients with renal disease. The institutional review board approved this study (No.2020060).
Clinical indicators
Epidemiological characteristics, including gender, age, season of onset, and precursor events. Clinical manifestations, including first symptoms, sensory disturbances, cranial nerves involvement, autonomic dysfunction, respiratory muscle palsy, weakened or disappeared tendon reflexes, time to peak deficit and severity of neurological deficits. The Hughes motor scale (HMS) is a function grading scale ranging from 0 to 6 (0—normal, 1—mild signs and capable of running, 2—capable of walking unaided for 5 m, 3—walking 5 m with support, 4—relying on bed or wheelchair, 5—requiring ventilatory support, 6—dead).5 The HMS at the worst muscle strength in the progression of the disease was evaluated. Those with HMS>3 were classified as the severe group, and those with HMS≤3 were classified as the mild group.
Auxiliary examinations
serum sodium, serum cysC, electrophysiological assessment, and CSF examination. A serum sodium <135 mmol/L was used as the diagnostic criterion for hyponatremia.6 The serum cysC concentrations in the kidney function test in our hospital were measured regularly on the morning of the second day of patient’s admission and detected by a latex particle-enhanced turbidimetric immunoassay (Mitsubishi Chemical Medience). The serum reference range of cysC was (0.50–0.96) mg/L. Electrophysiological parameters included motor and sensory nerve conduction, F wave abnormality (including prolonged latency and abnormal appearance rate), and conduction block. The electrophysiological classification used the diagnostic criteria from Hadden et al7 acute inflammatory demyelinating polyradiculoneuropathy (AIDP), acute motor axonal neuropathy (AMAN), acute motor and sensory axonal neuropathy (AMSAN) and ambiguous unclassified group.
Treatment
According to guidelines and actual conditions, patients received IVIG at 0.4 g/kg/day for 5 days. Routine neurological care during hospitalization, paying close attention to changes in the condition. Ventilatory assistance and plasma exchange (PE) were provided in the intensive care unit (ICU) as necessary.
Statistical analysis
The SPSS 22.0 statistical software was used, continuous variables were expressed as means±standard deviations or medians with interquartile ranges (IQR), and t test was used to compare the differences between 2 groups; categorical variables were expressed as the proportion of events, and Chi-square test was used to compare the rates between 2 groups. Logistic regression was used to analyze the independent risk factors of a poor functional outcome (HMS>3), and OR and 95%CI were calculated. The area under the ROC curve of combining serum cysC and respiratory muscle palsy or respiratory muscle palsy alone was calculated respectively. Statistical significance was defined as a two-tailed p-value<0.05.
Results
Clinical baseline characteristics
The study included 101 GBS patients, 59 males and 42 females (M: F=1.4:1), with an average age of 50.61±1.74 years. The incidence rate was 20.8% in spring, 30.7% in summer, 18.8% in autumn, and 29.7% in winter. Precursor events were seen 51 (50.5%) cases, including 31 cases of upper respiratory infection, 16 of diarrhea, two of shingles, one each of surgery and exogenous ganglioside application. 90 cases of electrophysiological assessments included 57 (63.3%) cases of AIDP, 17 (18.8%) of AMAN, 7 (7.7%) of AMSAN, and 9 (10%) of unclassified. There were 75 (74.3%) cases in the mild group, and 26 (25.7%) in the severe group including 5 (4.9%) who died (Table 1).
- Baseline characteristics and factors affecting GBS severity.
Factors affecting GBS severity
On univariate analysis, respiratory muscle palsy (p<0.001), time to peak deficit (p<0.001), hyponatremia (p<0.001), electrophysiological axonal pattern (p=0.005), IVIG therapy (p=0.004), and need for ventilatory support (p<0.001) were significantly associated with a poor functional outcome. There were no significant differences in age, gender, season of onset, precursor events, clinical symptoms, anti-ganglioside antibody, the rest of the electrophysiological assessment and CSF protein between the 2 groups (p>0.05) (Table 1).
The serum cysC levels were higher in the severe group than in the mild group, and the difference was significant [0.98 (0.85-1.20) vs. 0.81 (0.76-0.95), p=0.004] (Table 1) (Figure 1).
- The serum cysC value in Mild group vs. severe group.
Spearman rank correlation analysis of the Hughes motor scale and various factors. There was a positive correlation between the serum cysC and Hughes motor scale in GBS patients (r=0.376, p=0.001) (Table 2).
- Correlation of the Hughes motor scale and various factors.
Logistic regression analysis of the Hughes motor scale and various factors
On multivariate analysis, respiratory muscle palsy (OR 0.010, 95% CI 0.000-0.200, p=0.003), time to peak deficit (OR 0.686, 95% CI 0.503-0.936, p=0.017), serum cysC (OR 1.641, 95%CI 1.043-2.854, p=0.045) and hyponatremia (OR 0.784, 95%CI 0.644-0.954, p=0.015) were independent risk factors for a poor functional outcome (HMS>3) (Table 3).
- Logistic regression analysis of the Hughes motor scale and various factors.
Area under the ROC curve
The cut-off value of serum cysC is 0.875, which corresponds to a sensitivity of 0.769 and a specificity of 0.569.
Combining serum cysC and respiratory muscle palsy was more valuable for assessing disease severity than respiratory muscle palsy alone (AUC 0.863 vs. 0.787, p=0.001) (Figure 2).
- Area under the ROC curve.
Discussion
The GBS is a life-threatening disease with a mortality rate of 3–7%;8 patients die mainly of ventilatory insufficiency, pulmonary complications, or autonomic dysfunction. Analysis of the risk factors of GBS patients can help doctors to formulate treatment plans and judge the prognosis of patients. In this study of 101 patients, the ratio of male to female was 1.4:1, indicating male dominance. About half of the patients had a history of infection within 6 weeks before the disease, and most of the infections were upper respiratory tract or gastrointestinal infections. In our study, patients’ symptoms reached peak disability at an average of 7 (4–10) days, slightly less that described by Wang et al.9 and consistent with Paul et al.10
In terms of electrophysiology, two-thirds of the patients in this study were found to have a demyelinating variant, and an axonal variant that included AMAN and AMSAN was less but also a quarter. According to reports, 5% of GBS in North America and Europe are axonal variant, while this variant is much more common in Northern China, Japan and the rest of America.11 Patients with the axonal variant also had poor functional outcomes. Patients with AMAN have a more rapid progression of weakness to an earlier nadir than in AIDP resulting in prolonged paralysis and respiratory failure over a few days.12 The distinction seems clear conceptually, but the borders between the 2 conditions are not well defined. The electrophysiological findings are sometimes ambiguous, as they indicate AIDP in the early phases and AMAN later, or both conditions simultaneously.13
In our series, hyponatremia occurred in 17.2% of GBS patients, with a higher incidence in the severe group (38.5% vs. 9.6%, p=0.002). Hyponatremia is common in GBS patients14 and might be related to increasing age, concurrent malignancy, diuretic use, preceding diarrhea and IVIG treatment.15 Hyponatremia is an important predictor of a poor outcome in GBS. Therefore, the importance of hyponatremia when assessing the prognosis of patients with severe GBS should be emphasized. Clinicians should pay attention to not only the respiratory muscle palsy, but also the electrolyte levels in patients. Timely and accurate treatment can improve the prognosis.
The CysC is considered a housekeeping gene because it protects cells from hydrolysis by endogenous and exogenous proteolytic enzymes, and is the strongest cathepsin inhibitor known. CysC is secreted mainly from the choroid plexus into human CSF, where the concentration [(2.04–3.58) mg/l] is five times the serum level [(0.50–0.96) mg/l]. CysC in the CSF is an endogenous neuroprotective and plays a very important role in different neurological diseases, such as brain tumors, stroke and neurological autoimmune diseases.1 When inflammation and tumors occurred in the brain, cysC in the CSF entered the blood circulation through the pathologically altered blood-brain barrier (BBB), which in turn increases the serum cysC concentration, while the CSF cysC concentration decreases accordingly. Yang identified that cysC was the most significant differential protein among 6 protein spots in the CSF of GBS patients by using proteomic methods, and its downregulation in CSF proved its potential use in diagnosis and finding treatment.2 Li3 also reported that the accuracy of CSF cysC in the diagnosis of GBS was 0.827, although it cannot distinguish AIDP from AMAN. The decrease of cysC in CSF was also observed in CIDP and MS. A possible explanation might be that inflammatory cytokines down-regulate cysC secretion. Proteolytic enzymes like cysC are associated with demyelination and blood-CSF barrier dysfunction in GBS.16
Although blood is rarely in direct contact with the central and peripheral nervous systems, the circulation and renewal of CSF lead to an exchange of blood components. Then, the state of nervous system diseases in the body can be reflected by some blood components. Serum cysC levels can predict the extent of peripheral nerve damage. Diabetic patients with peripheral neuropathy have higher serum cysC levels than simple diabetic patients or healthy volunteers.17 In our study, serum cysC levels were significantly higher in the severe group than in the mild group, and were positively correlated with the degree of physical disability. The increase in serum cysC implies that it is involved in the pathology of GBS, and possible reasons are damage to the blood-brain barrier or blood-CSF barrier leads to the outflow of cysC from the brain;18 serum cysC is involved in regulating the cysteine protease activity and participating in inflammatory response;16,19 or the stress response of disease, including the secretion and synthesis of antidiuretic hormone (ADH) and renin-angiotensin, reduce the glomerular filtration rate, resulting in decreased excretion of cysC.20,21 There is an inflammatory response mechanism in the pathogenesis of GBS.22 Serum cysC is involved in the regulating the inflammatory response, which mainly affects neutrophil migration, phagocytosis, and chemotaxis. The higher serum cysC level in GBS patients is, the more severe the inflammatory, the greater edema of the nerve roots, and the greater the limb paralysis; however, the exact mechanism needs further study with other inflammatory factors.
There were some limitations in data availability due to a retrospective study. First, we included only 3-year inpatients from our hospital, and it was susceptible to selection bias and other confounding factors. Second, most GBS patients had only one electrophysiological assessment, and some are even absent, leading to errors in discriminating between axonal and demyelinating types. Third, HMS at the worst muscle strength in the progression of the disease was evaluated in our study, and long-term endpoints should be identified for more accurate predictions, such as 3-month outcomes.
This study found that serum cysC levels were positively correlated with the degree of limb paralysis in GBS patients, and serum cysC was a risk factor for a poor functional outcome (HMS>3) in GBS patients. Perhaps further study can observe the dynamic changes in cysC in serum and CSF during the development of the disease, with separate analysis of axonal and demyelinating subtypes, to better understand the mechanism of cysC in GBS.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received December 30, 2021.
- Accepted May 29, 2022.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.