Abstract
Objectives: To investigate the factors that contribute to the development of cerebral edema after aneurysm clipping in individuals with aneurysmal subarachnoid hemorrhage (aSAH).
Methods: A total of 232 patients with aSAH caused by rupture and treated with aneurysm clipping were included in the retrospective analysis of clinical data. Postoperatively, the participants were categorized into two groups based on the presence or absence of cerebral edema: a complication group (n=33) and a non-complication group (n=199).A comparison was made between the overall data of the 2 groups.
Results: In the complication group, there were higher proportions of patients experiencing recurrent bleeding, aneurysm in the posterior circulation, Fisher grade III-IV, World Federation of Neurosurgical Societies (WFNS) grade II, Hunt-Hess grade III-IV, concomitant hypertension, duration from onset to operation ≥12 h, and concomitant hematoma compared to the non-complication group (p<0.05). Cerebral edema after aneurysm clipping was associated with several risk factors including repeated bleeding, aneurysm in the back of the brain, Fisher grade III-IV, WFNS grade II, Hunt-Hess grade III-IV, simultaneous high blood pressure and hematoma, and a duration of at least 12 hours from the start of symptoms to the surgical procedure (p<0.05).
Conclusion: In patients with aSAH, the risk of cerebral edema after aneurysm clipping is increased by recurrent bleeding, aneurysm in the posterior circulation, Fisher grade III-IV, WFNS grade II, Hunt-Hess grade III-IV, concomitant hypertension and hematoma, and duration of ≥12 h from onset to operation.
Aneurysmal subarachnoid hemorrhage (aSAH) is a prevalent cerebrovascular occurrence characterized by the manifestation caused by the bursting of an intracranial aneurysm and the entry of blood into the subarachnoid space, which is the primary reason for SAH. The aSAH, which is marked by sudden onset and a significant death rate, presents itself as intense head pain, nausea, and neurological dysfunction.1 Over the past few years, there has been a gradual rise in the occurrence of aSAH. Surgery is the main treatment means for aSAH, especially aneurysm clipping which can effectively remove hematoma, restore cerebral blood circulation, and reduce the rebleeding risk, with low surgery cost.2,3 The exact cause of postoperative complications in patients with brain injuries caused by extensive aSAH after aneurysm rupture and trauma during surgery is currently unknown. The majority of patients experience cerebral edema after surgery, which can cause additional harm to nerve function and result in intracranial hypertension and cerebral hernia. This prolongs the recovery period and worsens the disease.4 Hence, it is highly important to investigate the factors that contribute to cerebral edema after aneurysm clipping in patients with aSAH and implement proactive measures for prevention and treatment to improve the prognosis of these patients. In order to gain a deeper comprehension of the factors that contribute to cerebral edema after surgery for aSAH, we conducted a retrospective analysis of the clinical information of aSAH patients who underwent aneurysm clipping at our hospital between May 2016 and May 2022. This study aimed to explore the associated risk factors for cerebral edema post-surgery, offering valuable insights for clinical prevention and treatment.
Methods
General data
This is a retrospective study. We retrospectively analyzed the clinical information of 232 patients with aSAH caused by rupture who received aneurysm clipping at our hospital between May 2016 and May 2022. The medical ethics committee of Peking University Shenzhen Hospital granted approval for this study, which was conducted in accordance with the guidelines outlined in the Helsinki Declaration. All patients provided written consent.
Criteria for inclusion and exclusion
Patients who met the diagnostic criteria for aSAH5 and were diagnosed by a head CT scanner (Canon, Tokyo, Japan), as well as those with intracranial aneurysm rupture confirmed by cranial digital subtraction angiography system (Siemens, Nuremberg, Germany) or CT angiography, and who underwent aneurysm clipping without any surgical contraindication, were included. Additionally, only patients with complete clinical data were considered. Patients who were excluded from the study included individuals with cerebral hemorrhage unrelated to aneurysm, those with intracranial pseudoaneurysm, those with spontaneous subarachnoid hemorrhage, those with coagulation abnormalities, and pregnant or breastfeeding women.
Methods
Operation method
Aneurysm clipping was performed on all patients. The location of aneurysm was identified by imaging examination. Then under general anesthesia, the subcutaneous and aponeurotic layers were incised, the flap was dissociated, the skull was drilled, and the bone flap was sawed and pried open. Afterwards, the microscope revealed the artery, allowing for identification of the anatomical location of the aneurysm, blood vessel, and nerve, followed by the separation of the aneurysmal neck. Wound hemostasis and washing were performed if necessary. Afterward, the suitable section of the aneurysm neck was clamped using a durable aneurysm clip. This was then followed by meticulous wound hemostasis and closure of the incision.
Postoperative cerebral edema was determined and classified by conducting a follow-up brain CT scan after the surgery. The presence of cerebral edema was characterized by the patient simultaneously experiencing increased intracranial pressure, expanded brain pathways, reduced depth of the cerebral cortical sulci, and decreased density and size of the brain parenchyma. Postoperatively, the patients were categorized into 2 groups based on the presence of cerebral edema: a complication group (n=33) and a non-complication group (n=199). The study retrospectively analyzed and compared various factors between the 2 groups, including age, gender, frequency of bleeding, location of aneurysm (in the anterior or posterior circulation), number of aneurysms (single or multiple), aneurysm diameter, modified Fisher grade of subarachnoid hemorrhage based on CT assessment,6 World Federation of Neurosurgical Societies (WFNS) grade of nerve function,7 Hunt-Hess grade,8 presence or absence of hypertension, diabetes mellitus, coronary heart disease, hematoma, and duration from onset to operation.
Statistical analysis
Statistical analysis was conducted using SPSS 22.0 software from IBM Inc. (Armonk, NY, USA). The measurement data were represented as (x±s) and compared using the independent-samples t test to analyze differences between the 2 groups. The count data were expressed as a percentage (%) and analyzed for intergroup comparison using the χ2 test. The variables were subjected to multivariate logistic regression analysis, and the concordance was determined using the Kappa test. A significance level of less than 0.05 was deemed statistically significant.
Results
Baseline clinical data
A total of 123 men and 109 women, ranging in age from 22 to 82 years old, had an average age of (51.37±5.48) years. After surgery, 33 cases suffered from cerebral edema and the other 199 did not. Consequently, they were segregated into a group with complications and a group without complications. No notable variations in gender, age, number of aneurysms, aneurysm diameter, and the existence or absence of diabetes mellitus and coronary heart disease were observed between the 2 groups (p>0.05) (Table 1).
- Results of univariate analysis on cerebral edema following aneurysm clipping [n (%)]
Results of univariate analysis on cerebral edema following aneurysm clipping. In the complication group, there was a higher occurrence of recurrent bleeding, aneurysm in the posterior circulation, Fisher grade III-IV, WFNS grade II, Hunt-Hess grade III-IV, concomitant hypertension, duration from onset to operation ≥12 h, and concomitant hematoma compared to the non-complication group (p<0.05) (Table 1).
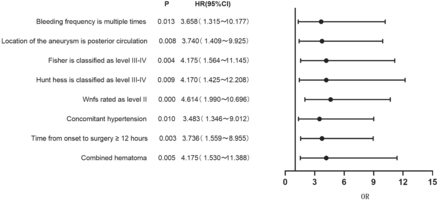
Results of multivariate logistic regression analysis on the occurrence of cerebral edema after aneurysm clipping. The multivariate logistic regression analysis included statistically significant variables such as cerebral edema, bleeding frequency, aneurysm location, Fisher grade, WFNS grade, Hunt-Hess grade, presence or absence of hypertension and hematoma, and duration from onset to operation. These variables were quantitatively assigned. The findings confirmed that repeated bleeding, aneurysm in the back part of the circulation, Fisher grade III-IV, WFNS grade II, Hunt-Hess grade III-IV, existence of high blood pressure and blood clot, and time from the start of symptoms to surgery being 12 hours or more were all factors that increased the risk of cerebral swelling after aneurysm clipping in patients with aSAH (p<0.05) (Table 2 & Figure 1).
- Forest plot of clinical characteristics based on multivariate logistic regression analysis.
- Results of multivariate logistic regression analysis on cerebral edema following aneurysm clipping.
Ability to predict the occurrence of cerebral edema after aneurysm clipping in patients with aSAH using a combination of risk factors. The concordance analysis utilized the clinical outcome as the golden standard. Positive combined prediction was determined by considering recurrent hemorrhage, aneurysm in the posterior circulation, Fisher grade III-IV, WFNS grade II, Hunt-Hess grade III-IV, presence of high blood pressure and hematoma, and duration of ≥12 hours from symptom onset to surgery as risk factors. Concordance analysis confirmed that the combination of risk factors accurately predicted cerebral edema after aneurysm clipping in patients with aSAH (Table 3). The sensitivity, specificity, accuracy, positive predictive value, negative predictive value, and Kappa value were determined to be 0.909, 0.980, 0.970, 0.882, 0.984, and 0.878, respectively.
- Predictive efficiency of combination of risk factors for cerebral edema following aneurysm clipping.
Discussion
The aSAH is a prevalent urgent situation in the field of neurosurgery, primarily triggered by the rupture of an intracranial aneurysm. If not promptly addressed, it poses a life-threatening risk to the patient and can result in neurological damage for those who survive.9,10 A commonly employed clinical approach to treat aSAH is aneurysm clipping, which effectively mitigates the condition and reinstates the circulation of blood flow in the brain. Nevertheless, the process of aneurysm clipping is intricate, and it can cause additional harm to the patient’s brain tissues, resulting in complications after the surgery.11,12 Cerebral swelling is a common complication that occurs after aSAH, and these patients are susceptible to increased pressure inside the skull, which affects their prognosis.13 Hence, it is crucial to investigate the factors that contribute to cerebral swelling after aneurysm clipping in aSAH patients and implement appropriate measures to decrease its occurrence.
A retrospective analysis was performed on 232 patients with aSAH in this study, which included 33 individuals who experienced cerebral edema following aneurysm clipping. Univariate analysis revealed that the complication group exhibited higher proportions of patients with recurrent bleeding, aneurysm in the posterior circulation, Fisher grade III-IV, WFNS grade II, Hunt-Hess grade III-IV, concomitant hypertension, duration from onset to operation ≥12 h, and concomitant hematoma compared to the non-complication group (p<0.05). The multivariate logistic regression analysis showed that recurrent hemorrhage, aneurysm in the posterior circulation, Fisher grade III-IV, WFNS grade II, Hunt-Hess grade III-IV, concomitant high blood pressure and blood clot, and a duration of 12 hours or more from symptom onset to surgery were identified as significant risk factors for cerebral swelling after aneurysm clipping in patients with aSAH (p<0.05).
Repeated bleeding following surgery will lead to a higher occurrence of postoperative cerebral edema in individuals with aSAH. This is because the absorption of human cerebrospinal fluid primarily occurs in the subarachnoid circulation.14 Spontaneous aSAH causes an elevation in hemoglobin levels, which in turn triggers inflammation and an increase in cerebrospinal fluid. In the meantime, hemoglobin can also trigger a change in the structure of the brain choroid plexus, worsening brain tissue damage and leading to swelling, resulting in disruptions in the circulation of cerebrospinal fluid.15,16 Patients are prone to developing hematoma after the rupture of an aneurysm in the anterior circulation, and the rupture and bleeding of an aneurysm in the arterial circle mostly occur in the ventricle and cistern, which can easily cause disruptions in the circulation of cerebrospinal fluid and cerebral swelling.
The Fisher grade indicates the extent of bleeding in imaging, and the higher the grade, the greater the degree of bleeding. Patients who have a high Fisher grade are prone to experiencing severe postoperative cerebral vasospasm. The severity of vasospasm is considered one of the major risk factors for cerebral edema after aSAH.17,18 The Hunt-Hess grade can indicate the level of consciousness and clinical symptoms in patients. A high Hunt-Hess grade suggests poor consciousness and severe brain tissue injury caused by SAH, which can disrupt cerebrospinal fluid circulation and increase the risk of cerebral edema.19,20 Additionally, the WFNS grade can help predict the neurological function and prognosis of patients, with higher grades corresponding to more severe injuries. Patients diagnosed with WFNS grade II exhibit the presence of cerebral edema.21 This occurrence is attributed to prolonged hypertension, which exerts increased pressure on the vascular wall, resulting in damage to the vascular endothelium and subsequent thickening and hardening of the vascular intima.22,23 The hardening of the vascular wall can lead to cerebral vasospasm, heightened cerebral blood perfusion, and an elevated likelihood of cerebral edema.24 Recent investigations have delved into the optimal timing for surgery in cases of aSAH, revealing that the longer the onset time, the more severe the postoperative cerebral edema tends to be.25,26 These findings align with the outcomes observed in the present study.
Moreover, the presence of intraventricular hematoma increases the likelihood of cerebral edema, particularly in patients with aSAH and intraventricular hemorrhage. Early drainage can help reduce the occurrence of cerebral edema. The clinical outcome was employed as the benchmark in the concordance analysis of this study, which verified that the amalgamation of risk factors yielded a sensitivity, specificity, accuracy, positive predictive value, negative predictive value, and Kappa value of 0.909, 0.980, 0.970, 0.882, 0.984, and 0.878, respectively, for the prediction of cerebral edema after aneurysm clipping in patients with aSAH. Patients with aSAH who have recurrent bleeding, aneurysm in the posterior circulation, Fisher grade III-IV, WFNS grade II, Hunt-Hess grade III-IV, hypertension and hematoma, and a duration of ≥12 hours from onset to operation are more likely to experience cerebral edema after aneurysm clipping.
To summarize, the occurrence of repeated bleeding, aneurysm in the back circulation, Fisher grade III-IV, WFNS grade II, Hunt-Hess grade III-IV, the existence of high blood pressure and hematoma, and a duration of ≥12 hours from the start of symptoms to the surgical procedure are all factors that increase the risk of cerebral swelling after aneurysm clipping in individuals with aSAH. To reduce the occurrence of cerebral edema after aneurysm clipping in patients with aSAH, it is important to closely monitor and intervene for such patients. However, there are limitations to this study. This is a retrospective study, so further prospective studies are in need to prove our conclusion.
Acknowledgement
We would like to thank all coauthors for their significant contributions to this study.
- Received August 23, 2023.
- Accepted December 18, 2023.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.