Abstract
The combination of vascular anomalies with gliomas is rarely seen in the CNS, and is defined as ‘angioglioma’. However, the definition, category, and histopathogenesis of angiogliomas remain controversial. Here, we present an unusual case of spinal hemangioblastoma (HB) combined with pilocytic astrocytoma (PA). Spinal MRI revealed lesions extending from T9 to T12 segments, in a “sandwich-like” fashion. After resection of the tumor, histopathologic study confirmed the diagnosis of HB as well as PA. A comprehensive review of the literature was further conducted. We describe a case of spinal HB combined with PA, in addition we discuss the clinicopathological relationship between HB and PA under these conditions, which may facilitate the understanding of the histogenesis of an angioglioma and guide its diagnosis and treatment.
The association of gliomas with certain types of vascular anomalies has been designated as angioglioma, and is rarely seen in the CNS.1 Councilman2 for the first time coined the term of “angiogliomas” to describe a highly vascular cerebellar neoplasm with features that were later recognized as the cellular variant of hemangioblastoma. Angiogliomas are roughly defined as mixed neoplasms composed of a low-grade glioma and a vascular anomaly. The combination of glial component and vascular component can be diverse. The vascular anomalies may be associated with various glial components, including fibrillary astrocytoma, pilocytic astrocytoma (PA), xanthoastrocytoma, and so on. Whereas the glial tumors may be associated with arteriovenous malformation, cavernous angioma, and hemangioblastoma (HB), more seen in the first 2 types.1,3 The incidence of angioglioma is relatively low as a previous study found only 2 cases in 168 low-grade gliomas.3 Compared with their intracranial counterparts, spinal cord angiogliomas are less common, let alone those presenting as HBs with astrocytomas. Our objective in presenting this particular case is to report a spinal HB combined with PA extending from T9 to T12 levels, complicated by an extensive syrinx. The lesions were in a “sandwich-like” fashion both in MRI and surgical findings, and exhibited particular histopathologic characteristics. We also review the related literature and discuss the features related to this special type of angioglioma.
Case Report
Clinical course
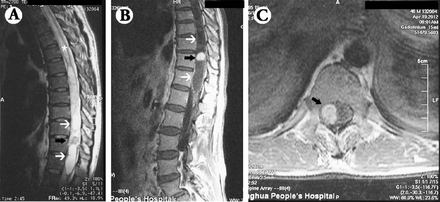
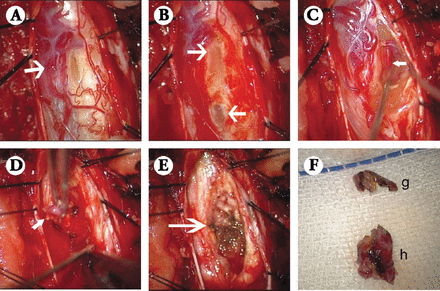
A 47-year-old man was admitted to our hospital, presenting with progressive numbness, and weakness of the right lower extremity over one month. He had no headache, vomiting, ataxia, or sphincter dysfunction. Physical examinations revealed hyperesthesia and weakened muscle strength in the right lower extremity, and no history of systemic diseases or genetic diseases were found. Preoperative MR imaging of the spinal cord revealed multiple lesions (Figure 1). Two cystic, sharply demarcated intramedullary lesions were located from T9 to T12 segments, hypointense on T1WI, hyperintense on T2WI, and slight enhancement of the margins after gadolinium injection. Interestingly, they were separated by a nodule that was both hypointense on T1WI and T2WI, but intensely enhanced after gadolinium administration. These lesions presented in a “sandwich-like” fashion, as the nodule was enclosed by the cysts on its 2 sides. A long segment syrinx was also demonstrated on T2WI throughout the whole thoracic cord. The abdominal ultrasound scan was also performed, which revealed no signs of renal cyst, pancreatic cyst, or any other lesion. Surgical resection of the T9-T12 intramedullary spinal cord tumors (ISCT) was performed (Figure 2). A superficial pial component of the soft tumor was identified with marked tortuous draining veins (Figure 2A). The 2 cysts were sequentially resected and drained (Figures 2A & 2B). Deeper vascular anomalies were further identified and resected (Figures 2C & 2D). The capsule of the cysts and the vascular conglomeration were sent to the pathological department (Figure 2F).
Patient MRI showing: A) T2WI showed 2 hyperintense lesions (white arrows) separated by a hypointense nodule (black arrow) from T9 to T12 levels. The syrinx extending the whole thoracic segments was shown (asterisk). B) T1WI enhancement showed homogeneous enhancement of the middle nodule, while its bipolar lesions were not enhanced. C) Axial enhanced scan showed an enhanced lesion in the spinal canal (black arrow).
Microsurgical view of the lesions during operation showing: A) Large draining veins on the dorsal and dorsolateral spinal cord surface were seen with the opening cyst after dural opening. B) Two separated cysts were opened, and light yellow fluid flowed out. C) A large vascular anomaly was exposed deeper inside the spinal cord (white arrow). D) The vascular conglomeration was resected in the spinal cord (white arrow). E) Cauterized and Gelfoam placement for hemostasis after successful resection of the tumors. F) Resected specimens for pathological studies, one was part of the cystic capsules (g), and the other was the vascular conglomeration that measured approximately 0.5×0.5×0.5cm in size (h).
Histopathologic and immunohistochemical findings
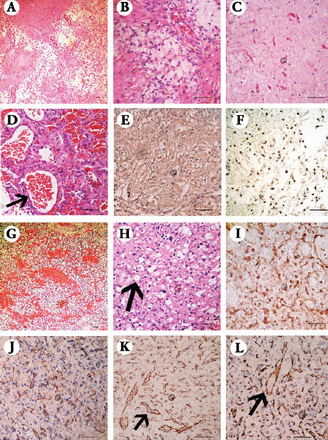
The tumor specimens were investigated for histopathological studies. The capsule specimens, in Hematoxylin and Eosin (H&E) staining, demonstrated a typical biphasic pattern with varying proportions of compacted bipolar cells associated with Rosenthal fibers and loose-textured multipolar cells associated with microcysts and hyaline droplets (Figure 3). The compact portions of the tumor yielded bipolar piloid cells with long and hairlike processes (Figures 3A & 3B). The microcystic areas possessed round to oval, cytologically bland nuclei and relatively short, cobweb-like processes that were fibril-poor (Figure 3B). The Rosenthal fibers were observed as tapered corkscrew-shaped, brightly eosinophilic, hyaline masses (Figure 3C). The abundant glomeruloid vasculature indicated the highly vascularized feature (Figure 3D). The pathologic features were consistent with PA, WHO grade I. In the specimens of the vascular conglomeration, H&E staining revealed 2 main components: stromal cells that were characterized by large lipid-containing vacuoles, and abundant vascular cells in accordance with the features of HBs. Further immunohistochemical (IHC) studies were conducted. In the capsule specimens, the glial fibrillary acidic protein (GFAP) was positive in the compact region in accordance with the high content of refractile, eosinophilic fibrils of bipolar piloid cells (Figure 3E). In the specimens of vascular conglomeration, S-100 and CD56, representing neuron-specific enolases or neural cell adhesion molecules, were all positive in the area of stromal cells (Figures 3I & 3J). In contrast, the VIII factor and the endothelium-associated adhesion molecule CD31 were all positive in the zones rich in endothelial cells (Figures 3K & 3L).
Microscopic view of the tumor with Hematoxylin and Eosin and immunohistochemical stainings showing: A) Biphasic pattern with varying proportions of compacted areas and loose-textured areas (×100). B) Biphasic pattern with compact portions yielding bipolar piloid cells, while the loose-textured areas possessed round to oval, cytologically bland nuclei and relatively short, cobweb-like processes, which are fibril-poor (×400). C) The Rosenthal fibers were observed as tapered corkscrew-shaped, brightly eosinophilic, hyaline masses (×400). D) The abundant glomeruloid vasculature (×400). E) The glial fibrillary acidic protein was positive in the compact region while negative in the loose-textured area (×400). F) The p53 was positive in the area of pilocytic astrocytoma (×400). G) Two components of stromal cells and abundant vascular cells (×100). H) Stromal cells that were characterized by large lipid-containing vacuoles (×400). I) S-100 was positive mainly in the area of stromal cells (×400). J) CD56 was positive mainly in the area of stromal cells (×400). K) the VIII factor was mainly positive in the area rich in endothelial cells (×400). L) CD31 was mainly positive in the area rich in endothelial cells (×400).
Discussion
Our case represented an unusual type of ISCT with combined components. The “sandwich-like” fashion illustrated on MRI was consistent with surgical findings, and was finally revealed by pathologists to be the combination of PA and HB. The 2 outer layers of the “sandwich” were both PA components, whereas the inner layer of the “sandwich” was HB component. Spinal PAs are the most common ISCT in the pediatric and adolescent population. They always grow insidiously, and symptoms can take months to years to evolve.4 Spinal HBs are relatively rare, comprising 2-15% of ISCTs.5 They can develop spontaneously or as a part of Von Hippel-Lindau (vHL) syndrome, which is an autosomal dominant neoplastic syndrome affecting multiple organ systems.5 In our case, considering the negative family history and absence of systemic diseases, sporadic HB was suggested. The HBs mainly arise in the dorsal root entry zone and grow in an intramedullary fashion, which was confirmed during the surgery.
The combination of HB and astrocytoma has existed in other sporadically reported cases on review of the literature.1,3 Bonnin et al6 reported a series of 4 patients with HBs and gliomas. The glioma types vary from reactive peripheral astrocytoma to mixed gliomas. A 65-year-old woman was reported to have both HB and mixed glioma (oligodendroglioma, ependymoma, and astrocytoma) in the cervical-thoracic segment of the spinal cord. However, only myelogram and CT scan were evaluated at that time, lacking detailed description of the lesion on MRI scan. Later, Lombardi et al7 reported 3 lesions of the type described by Bonnin et al6 in their review of 98 HBs and 125 cerebellar astrocytomas. However, no detailed descriptions could be obtained. Matyja et al1 in 2007 reported a series of cases with HB accompanied by advanced astrogliosis of adjacent brain tissue, including one specimen recognized of HB and PA. Nevertheless, because of their simply pathological study using formalin-fixed specimens, clinical information could not be obtained.
We report a case with HB and PA in the spinal cord, with comprehensive clinical and pathological information. In most of the previous angioglioma cases, the combined features were purely shown on histopathological studies by specimens from the same mass. Our case presented with a “sandwich” structure, representing the 2 different components, both in MRI and surgical findings. The final diagnostic specimens were obtained from neighboring but different surgical areas.
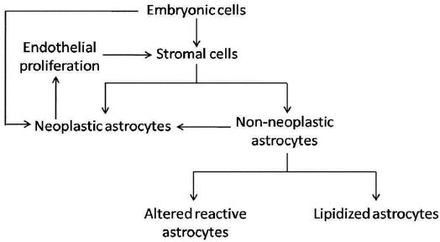
Various influencing factors have been suggested for the pathogenesis of angiogliomas, including genetic predisposition, reactive or malformative nature, viral origin, and exceptional coincidence.3 The histogenesis of HB combined with PA is rather complicated. Three related hypotheses have been postulated: 1) Some HBs induce reactive glial proliferation that eventually progresses to neoplasia; 2) The vascular component of a glioma with prominent endothelial proliferation undergoes secondary neoplastic change, developing into a HB; 3) Simultaneously or consecutively acting oncogenic factors determine the neoplastic transformation of both glial and angiogenic components, with concurrent participation of both the parenchyma and the stroma in the neoplastic process, resulting in a mixed tumor. The summary of these hypotheses is illustrated in Figure 4.
The illustration of hypotheses on the association of hemangioblastoma and pilocytic astrocytoma.
Regarding our case, the predominant hypothesis is yet to be established. The HB element was located centrally and was enclosed by 2 cysts of PAs in the “sandwich,” which seemed to support the view that the astrocytoma components resulted from dysregulated reactive astrogliosis growing in an eccentric pattern around the HB component. In fact, in the brain, tissue adjacent to the HBs could exhibit a more or less advanced astroglial reaction, which is predominant of the pilocytic type with numerous Rosenthal fibres.1 Parts of the stromal cells in HBs have been found to be GFAP-positive. Some of the GFAP-positive cells were identified to be neoplastic but lipidized or altered reactive astrocytes, some were neoplastic astrocytes, still others were stromal cells capable of taking up extracellular GFAP derived from the adjacent reactive astrocytes (Figure 4). Notably, surrounding chronic lesions in the spinal cord, the hairlike piloid cells of PAs are remarkably similar to reactive astrocytes, which serve as the prime candidates as precursors for piloid cells.8 The glial proliferation could be secondary to tissue changes after chronic ischemia and gliosis.3 In consideration of the histogenesis of gliotic tissue mentioned above, it is significant to distinguish true neoplastic astrocytes from the reactive astroglial cells. Three pieces of evidence point to the establishment of the diagnosis as PA rather than reactive gliosis: 1) The preoperative MRI indicated that the 2 cystic, intramedullary lesions had a clear margin, which was verified during surgery later. In comparison, the foci of reactive gliosis are not as discernibly demarcated as those of PA in most cases; 2) Although Rosenthal fibers might be detected in gliotic tissue, such as reactive gliosis and walls of cysts associated with nonglial tumors, they are characteristically contained in an abundant way in cystic areas of pilocytic astrocytomas, which were observed in our case. 3) Distinction between reactive gliosis and PA may be difficult by histology alone. Immunohistochemistry for some tumor-associated antigens, including p53, Bcl-2, and epidermal growth factor receptor (EGFR) and so forth, can be helpful in solving this problem. The lack of p53 immunoreactivity in reactive gliosis has been reported in a previous study.9 In contrast, p53 positive was found in PA, which allowed for the definitive diagnosis of PA versus reactive gliosis in our case (Figure 3F).
The second hypothesis might also come into play in the histogenesis (Figure 4). The proliferated endothelial cells in gliomas are capable of either being neoplastically transformed or are induced intrinsically neoplastic. The fluid content of cysts in PAs are rich in factors capable of stimulating vascular proliferation. Given the small portion of HB component in MRI as well as surgical findings, and slight marginal enhancements of the cysts, the HB element may result from merging of the neoplastic changes of endothelial cells in the separated astrocytoma components, by growing toward each other. Furthermore, it might originate from neoplastic endothelial cells at the center of a large PA and further disassociate into 2 parts. However, given the additional theory on the origin of HBs, we are in favor of the first hypothesis that HB component plays a leading role in our case. In HBs, the stromal cells represent the neoplastic component. It is postulated that embryonic progenitor cells with hemangioblastic differentiation potential represent a likely cytologic equivalent of the stromal cells.8 The embryological distribution of the subset of hemangioblasts within the developing brain is consistent with the observed locations of these tumors within the CNS. It is inferred that the distribution of HBs may result from developmental patterns rather than as a consequence of the migration of tumor cells.5
The prognosis of angiogliomas depends on several factors, such as the intrinsic biological behavior, location, amenability, extent of resection, treatment modality, and so on. The biological behavior of angiogliomas differs in no way from that of the similar grade gliomas unassociated with an excess vasculature. The 5- and 10-year survival rates of patients with only gliomas are entirely comparable to those of the corresponding angiogliomas. As a result, the term “angioglioma” was suggested to be of no diagnostic or prognostic importance since patient survival mainly depended on the histological type and grade of the associated glioma.
In conclusion, this report describes a case of spinal HB combined with PA in the T9-12 levels, also complicated by a long segment of syringomyelia. Our case typically presented a “sandwich-like” structure on MRI and surgical findings. The outer 2 layers and the inner layer respectively represent different tumor components on further pathological studies. We infer that the astrocytoma-component may be secondary to HB and the histogenesis of this kind of mixed tumors deserves further exploration.
Acknowledgments
The authors thank Neurosurgeon Dr. Yi-Cheng Lu, Pathologist Dr. Huimin Liu, and Radiologist Dr. Tao Jiang for their comments.
Footnotes
Disclosure
Authors declare no conflicting interests, support or funding from any drug company.
- Received April 9, 2014.
- Accepted March 20, 2015.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.