Abstract
Human immunodeficiency virus (HIV) infection associated aneurysmal vasculopathy is a rare complication of HIV infection affecting the pediatric and adult population. We present a case of a 7-year-old male child known to have a congenitally acquired HIV infection presenting with a ruptured left distal internal carotid artery fusiform aneurysm that was diagnosed on MRI scans 6 months prior to his presentation. He underwent craniotomy and successful aneurysm reconstruction. He had uncomplicated postoperative course and experienced a good recovery. This case is among the few reported pediatric cases of HIV-associated cerebral arteriopathy to undergo surgery. We also reviewed the relevant literature of this rare condition.
Neurological involvement frequently complicates the course of human immunodeficiency virus (HIV) infection in both pediatrics and adults.1 Cerebrovascular complications are rarely encountered in these setting and are attributed either, to the primary HIV infection or secondary complications of immunodeficiency.2,3 The incidence of cerebrovascular events in pediatric patients with HIV infection is estimated to be 3.4 cases per 10000 person-years.4 Human immunodeficiency virus–associated arteriopathy encompasses several forms of arterial diseases occurring in the absence of any cause other than HIV infection. The HIV–associated aneurysmal arteriopathy is a rare cerebrovascular complication of acquired immune deficiency syndrome (AIDS) and has been the subject of several case reports and case series involving children, and more recently adults, with AIDS.2,3,5-10 This aneurysmal arteriopathy is characterized by multiple, diffused aneurysmal dilatations confined to the major arteries of the circle of Willis.1,11 The mechanism by which HIV results in CNS arterial damage is still not clearly understood.2 Most patients with this condition present with cognitive changes and motor deficits associated with infarction or hemorrhage.12 Our objective in presenting this particular case is to report a documented de novo left fusiform carotid bifurcation aneurysm in a child with maternally acquired-HIV infection who presented with subarachnoid hemorrhage and underwent surgical clipping of the aneurysm.
Case Report
A 7-year-old boy who was known to have congenital HIV infection presented with a sudden onset of severe headache and altered level of consciousness of 2 days duration. Two years earlier, he tested positive for HIV infection when he presented with severe dyspnea and cough due to pulmonary tuberculosis. His HIV-1 RNA quantitative polymerase chain reaction (PCR) was carried out and showed 15042 copies/ml. Brain MRI and angiography (MRA) studies during that admission showed no abnormal intracranial or vascular findings (Figure 1). The CSF analysis revealed normal cell count, glucose and protein in a normal range, and negative latex agglutination test and culture. He had a remarkable recovery and was discharged on an 18-month course of anti-tuberculosis therapy and antiretroviral therapy: zidovudine, lamivudine, and lopinavir/ritonavir combination. He presented to the emergency department 18 months after with a new onset of generalized tonic-clonic convulsion. He was not compliant to antiretroviral therapy, and the HIV-1 RNA quantitative PCR showed a higher viral load of 310798 copies/ml. Brain MRI and MRA scans showed a 10 × 8 mm fusiform aneurysm in the left distal internal carotid artery (ICA) (Figure 2). He was managed with antiretroviral therapy and monitoring of the aneurysm with MRI after 3 months. At the current presentation, 6 months after, his general examination revealed low-grade fever (37.8°C), and his Glasgow coma score (GCS) was 14. There was neck stiffness, positive Kerning’s and Brudzinski’s signs but no papilledema or any focal neurological deficit.
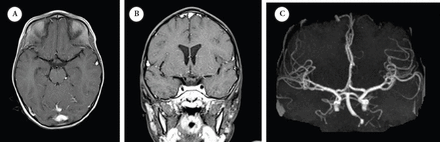
Initial presentation study A) post-contrast T1-wighted MRI and B) at the level of the Circle of Willis and maximum-intensity projection reconstruction MRI image C) demonstrating normal flow void and vascular anatomy at the circle of Willis, particularly at the left ICA and MCA. MCA - middle cerebral arteries, ICA - internal carotid artery
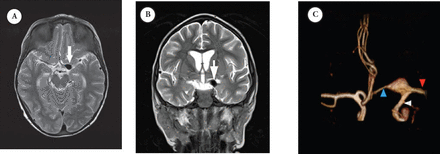
Second presentation MRI study: selected axial A) and coronal B) T2-weighted MRI at the region of circle of Willis showing an aneurysm at the junction between the distal ICA, M1-segment of MCA and the A1-segment of ACA, demonstrated as a flow void. This was well demonstrated with a C) 3D-reconstruction of the MRA. ICA - internal carotid artery, MCA -middle cerebral arteries, ACA - anterior and middle cerebral arteries
Early and confirmed diagnosis
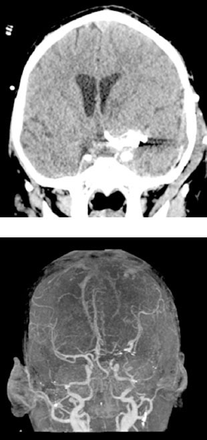
Brain CT scan revealed left Sylvian hyperdensity consistent with subarachnoid hemorrhage (SAH) with associated hydrocephalus (Figure 3A). The MRI scan demonstrated subacute blood products in the left Sylvian fissure with intraventricular extension and hydrocephalus. No basal leptomeningeal enhancement was noted on post-contrast images. Brain MRA scans demonstrated that the previously visualized fusiform aneurysm increased in size and included the proximal segments of the anterior and middle cerebral arteries (ACA and MCA). The chest x-ray and CT scans of the chest and abdomen were within normal limits. He was admitted to the ICU and an external ventricular drain (EVD) was inserted. The CSF analysis showed 31 WBCs, 91% of which were lymphocytes. Otherwise, CSF was unremarkable. He had remarkable improvement of his level of consciousness. Subsequent aneurysm delineation with CT angiography (CTA) was carried out and revealed a large 13 mm x 16 mm fusiform aneurysm at the left ICA bifurcation, which involves the proximal segments of ACA and MCA (Figure 3B). Investigations ruled out concurrent infections such as cytomegalovirus, Epstein-Barr virus, or varicella.
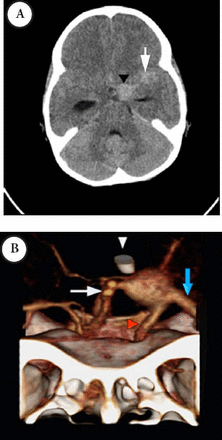
Third presentation CT study selected axial non-contrast enhanced CT of the brain A) showing interval increase in the dimensions of the aneurysm and SAH due rupture of the aneurysm. There was hydrocephalus seen as marked dilatation of the temporal horns of the temporal horns of the lateral ventricles. 3D-reconstruction of a contrast-enhanced CTA B) after insertion of EVD, demonstrating the large fusiform aneurysm at the junction between the distal ICA, M1-segment of MCA and the A1- segment of ACA. The bony landmarks of the cranial fossa and the anterior cloned process are also shown. MCA - middle cerebral arteries, ICA - internal carotid artery, EVD - external ventricular drain, ACA- anterior and middle cerebral arteries, CTA - CT angiography, SAH - subarachnoid hemorrhage
Treatment
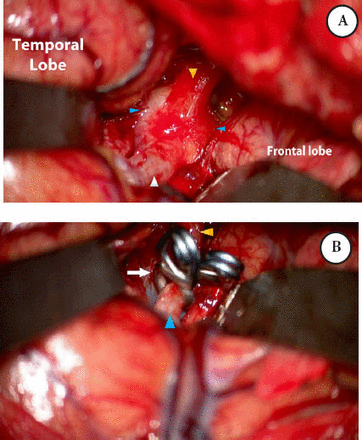
He underwent left pterional craniotomy and successful reconstruction of the aneurysm at the ICA/MCA using 2 fenestrated aneurysm clips and sacrificing the proximal part of the ACA. A clip of the remaining aneurysmal ACA was applied proximal to the takeoff of Huebner’s artery (Figure 4).
Intraoperative microscopic photograph through A) left frontotemporal craniotomy and transsylvian approach demonstrating the extent of the aneurysm, B) Repair of the fusiform ICA/MCA aneurysm was reconstructed with 2 fenestrated aneurysm clips. MCA - middle cerebral arteries, ICA - internal carotid artery.
Follow-up
He had an uneventful postoperative period. He could not be weaned of EVD, and required the insertion of a ventriculoperitoneal shunt. His postoperative CTA revealed adequate ICA/MCA reconstruction (Figure 5). He was discharged on a highly active anti-retroviral therapy (HAART) regimen of zidovudine 9 mg/kg by mouth, twice a day (PO BID), lamivudine (3TC) 4mg/kg PO BID, and Kaletra® (lopinavir/ritonavir) 15mg/kg PO BID.
Selected coronal non-contrast CT image of the brain A) showing the clip applied across the aneurysm. Maximum-intensity projection reconstruction B) and 3D reconstruction of the postoperative contrast-enhanced MRA, demonstrates the reconstruction of the aneurysm and the patent distal circulation.
Discussion
Cerebral vasculopathy has been described as an unusual manifestation of AIDS in several case reports and case series in pediatric and adult medical literature.13 Kure et al in 1989,14 reported the first case of HIV-associated cerebral aneurysmal arteriopathy in a pediatric patient. Since then, at least 23 reports (44 cases) of pediatric patients with HIV-associated cerebral aneurysmal arteriopathy have been published. These are summarized in Table 1.
Summary of all reported pediatric cases of HIV-related aneurysmal vasculopathy.
The HIV-positive pediatric patients have an increased incidence of cerebral aneurysms. Aneurysms associated with HIV infection are typically fusiform in shape.11 However, saccular aneurysms secondary to HIV infection have been reported in the literature.13,15 As in our case of a large fusiform aneurysm of ICA bifurcation with involvement of the proximal MCA and ACA, most of the cerebral aneurysms associated with HIV infection are located in the arteries of the circle of Willis.1-3,13,16
The HIV-associated cerebral vasculopathy usually presents with cognitive changes and motor deficits secondary to infarction or hemorrhage.2,15 Most patients with cerebral aneurysms secondary to HIV in the reported cases presented with seizures, headache, localized neurological deficits, and fatal subarachnoid hemorrhage.1 Cerebral aneurysms were reported as an incidental finding in asymptomatic patients with HIV-infection.5,13,17
The classical microscopic findings of autopsy examination of patients diagnosed with HIV-associated vasculopathy include medial fibrosis with the loss of the muscularis, destruction of the internal elastic lamina, and intimal hyperplasia.14,18 Many mechanisms were proposed in the literature to explain how HIV infection results in CNS arterial damage.1,2,11 The exact mechanism is still undefined.16,17 Whether cerebral vasculitis is directed primarily to HIV infection or secondary to associated infections such as varicella zoster virus, cytomegalovirus, or other opportunistic infections is still a matter of debate.1,2,15 Direct HIV invasion of the endothelium of cerebral arteries is one of the mechanisms that may explain the vasculopathy.16 The transmigration of certain HIV-infected monocytes through the blood-brain barrier during the phase of neural tissue invasion and the release of local and systemic toxins are among the proposed mechanisms.16 With the known tropism of those monocytes to the brain tissue, this might also explain the isolated cerebral vasculopathy in the absence of systemic vascular involvement.13,16
Petropoulou et al,17 reported the development of the cerebral aneurysm during a period with high circulating viral load. In the current case, we demonstrated the same observation as our patient was discovered to have the aneurysm at the time with his higher reported viral load (310798 copies/ml) and while he was not adhering to his antiretroviral medications. The high viral load is associated with the viral activity and could lead us to support the theory of a direct relation of the virus activity to the formation of these aneurysms. Husson et al13 reported a case with a formation of cerebral arteriopathy during a period of high p24 antigen levels signifying high viral replication. Kure et al14 has positively stained the cells of the wall of the affected arteries with antibodies against HIV transmembrane glycoprotein (gp-41) thorough immunohistochemistry. Dubrovsky et al2 and Mahadevan et al16 used PCR to amplify HIV-1 DNA from the dilated arteries of their reported cases. These observations support the direct contribution of the virus to the pathogenesis of the arteriopathy. Bonkowsky et al, in 2002,11 reported a case that presented during the immune reconstitution status. The CNS event occurred 5 months after the initiation of HARRT. Demopolous et al, in 2009,18 reported another 3 cases that presented after an average of 6 months of initiation of HAART.18 Both reports reported the clinical manifestations while the number of CD4 lymphocytes was increasing, and the HIV viral load was decreasing. The immune reconstitution inflammatory syndrome could be implicated in the pathogenesis of HIV-associated arteriopathy.18
The occurrence of aneurysmal vasculopathy during periods of higher viral load and profound immunosuppression has led investigators to consider the possibility of the presence of infective etiology acting in synergy with HIV.16 Associations between infections with varicella zoster virus, cytomegalovirus, and herpes simplex virus encephalitis with the development of the aneurysmal vasculopathy were reported in the literature.2,3,13 Our patient tested negative for active varicella zoster virus and cytomegalovirus infections through serum immunoglobulin.
The optimal management of patients with cerebral aneurysmal arteriopathy is still not well established.1 Older case reports, before the initiation of the highly active anti-retroviral therapy (HAART), have showed fatal outcomes in most patients either due to stroke or severe intracranial hemorrhage.2 While some reports have demonstrated ongoing progression of the disease despite the initiation of HAART, others have shown a stop of the disease progression,11,15,17 and even resolution of the arteriopathy after the initiation of HAART.1 These reports of improvement of arteriopathy after the initiation of HAART can also support the hypothesis of the direct role of HIV infection to the causation of this condition.16 Surgical intervention is rarely reported in pediatric patients with HIV-associated cerebral aneurysmal arteriopathy. The diffuse distribution and fusiform architecture of most of the aneurysms reported have hindered neurosurgical or endovascular treatment.
In conclusion, HIV-associated vasculopathy is a rare complication of HIV affecting pediatrics and adults. Cerebral aneurysms secondary to HIV-associated arteriopathy have been reported as an incidental radiological finding while most patients reported symptoms ranging from mild headache to sudden death secondary to subarachnoid hemorrhage. The early initiation of HAART seems to decrease the incidence of aneurysms and other cerebrovascular complications of HIV infection. Secondary etiologies, most importantly VZV vasculopathy, should be considered in the differential diagnosis of stroke and other cerebrovascular abnormalities in patients with HIV-infection. Appropriate investigations with early treatment for secondary etiologies should be pursued to decrease the complications of vasculopathy. Surgical and endovascular management are appropriate options when indicated, for both adults and pediatric patients with HIV-associated aneurysmal arteriopathy.
Footnotes
Disclosure
The authors declare no affiliation or financial involvement with organizations or entities with a direct financial interest in the subject matter or materials discussed in the manuscript. No funding was received for this work from any organization.
- Received January 27, 2015.
- Accepted May 11, 2015.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.