Abstract
Objectives: To assess the correlation between craniovertebral junction (CVJ) abnormalities and syringomyelia in patients with Chiari malformation type-1 (CM1).
Methods: This was a retrospective study including patients with CM1. Identification of cases was done by searching a radiology database at a university hospital from 2012 to 2017. Patients were divided into 2 groups based on whether CVJ abnormalities were present (CVJ+) or absent (CVJ-). The patients’ demographic and clinical data were reviewed. All magnetic resonance imaging studies were examined by a certified neuroradiologist.
Results: Sixty-four consecutive patients with CM1 were included. The mean age was 24±17 years; 59% were females. The CVJ+ group had more female patients (p = 0.012). The most frequent CVJ abnormality was platybasia (71%), followed by short clivus (44%) and cervical kyphosis (33%). The CVJ abnormalities were more in Syringomyelia cases (p = 0.045). However, the results were not significant when hydrocephalus cases were excluded.
Conclusion: Among CM1 patients, CVJ abnormalities were found more in patients with syringomyelia. Future studies with larger sample size are required to further study the correlation between CVJ abnormalities and both syringomyelia and hydrocephalus in CM1 patients.
Chiari malformation type-1 (CM1) was first described in 1891 by Austrian pathologist Hans Chiari.1,2 The CM1 is defined as caudal displacement of the cerebellar tonsils below the foramen magnum by 5 mm or more.3,4 This definition is merely a radiological definition. In the literature, the degree of cerebellar tonsil displacement varies from 3 mm to 5 mm.4 CM1 affects approximately 1% of the population and may involve a spectrum of neurologic involvement.2 Syringomyelia is reported in 25% of CM1 cases and may cause irreversible damage to the spinal cord with subsequent neurological deficits.5
The pathophysiology of syringomyelia development in patients with CM1 has been extensively studied.6-9 Majority of publications indicated a block to the cerebrospinal fluid (CSF) circulation at the level of the craniovertebral junction (CVJ).8,9 Subsequently, the cerebrospinal fluid (CSF) accumulates and forms syringomyelia.8,9 The source of the CSF forming the syringomyelia can be from the fourth ventricle, the subarachnoid space (SAS), or from an extracellular source.8,9 From the 1950s to the 1970s, syringomyelia was believed to result from a difference in CSF pressure between the fourth ventricle and the central canal of the spinal canal.7 Theories to explain this mechanism include James Gardner’s water-hammer theory, Bernard Williams’ cranio-spinal pressure dissociation theory, and Ball and Dayan’s theory of tonsillar obstruction to the CSF pathway.10-12 In the 1990s, Oldfield believed that the mechanism of the development of syringomyelia involved abnormal CSF flow at the level of the foramen magnum.6,7 The descent of the cerebellar tonsils with each cardiac cycle produces a pressure wave in the spinal SAS, and thereby compresses the spinal cord from the outside and propagates a syrinx.7,9
Several intradural and extradural factors have been implicated in the pathophysiology of CM1. Among the intradural factors identified during surgery for CM1, the presence of an arachnoid membrane obstructing the foramen of Magendie (i.e., an arachnoid veil) was significantly more frequent in patients with an associated syringomyelia.6 Other studies have examined whether the degree of tonsillar descent below foramen magnum in the CM1 patients is a contributing factor to the development of syringomyelia; however, the impact of tonsillar descent is controversial.6,9,13 Some studies have reported that the rate of syringomyelia increases as the degree of tonsillar herniation increases.6,9 As a possible explanation for syringomyelia development, other studies14,15 have addressed crowding of the SAS at the foramen magnum caused by tonsillar decent. In a study by Doruk et al15, the measured cervicomedullary compression ratio, defined as the ratio of the area occupied by the cerebellar tonsils to the area of the foramen magnum, was significantly correlated with the development of syringomyelia. This ratio could reflect the severity of blockage of the SAS at the CVJ and further supports the previously described mechanisms of syringomyelia development.9
Extradural abnormalities at the CVJ are associated with CM1.15 Such pathologies include a small posterior cranial fossa, platybasia, basilar invagination, and short clivus.3,6,8,9 Several studies have examined the presence of CVJ abnormities in CM1 patients with and without syringomyelia.13,16-21 However, the presence of associated syringomyelia within the context of CM1 with and without CVJ abnormalities was inadequately highlighted. For instance, in one study,13 syringomyelia existed in 64% of CM1 patients with a short clivus, compared to 36% of CM1 patients without a short clivus. In order to further understand the relationship between the presence of one or more CVJ abnormalities and syringomyelia in CM1, the current study was conducted. Such knowledge will likely enhance the understanding of CVJ relationship with CM1 and may aid in the management of syringomyelia in such patients.
Methods
Basic study design
This was a retrospective study. We conducted an electronic search for all cases of Chiari malformation (CM) through the Imaging Picture Archiving and Communication System (PACS) database at a university hospital in Riyadh, Saudi Arabia. All consecutive patients diagnosed with CM from 2012 to 2017 were identified. Only CM1 cases, defined as the displacement of the cerebellar tonsil equal or more than 5 mm below the foramen magnum, were included.3,4 We excluded patients who did not meet the criteria for tonsillar decent or patients with underlying infection or tumors affecting the brain or the spinal cord. The study protocol was approved by the institution’s review board.
Radiologic parameters and analysis
Magnetic resonance imaging (MRI) studies of all included patients were carefully reviewed by a certified neuroradiologist who was blinded to the clinical data. The included radiological parameters were selected based on previously published data describing their relationship to CVJ abnormalities or to CM. The mid-sagittal T1-weighted view MRI was used to assess the CVJ for the following radiological parameters:
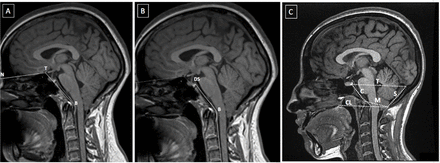
The NTB angle of Welcker (Figure 1A): The angle formed by the line extending from the nasion (N) to the tuberculum (T) and the line extending from the tuberculum to the basion (B). Platybasia is marked by an increased NTB angle more than 130°.22
Demonstration of the included anatomical methods to evaluate craniovertebral junction abnormalities. A) points of the NTB angle of Welcker, B) the clivus canal angle, and C)clivus length, supraocciput line (S), McRae’s line (M), Twining’s line (T), and Chamberlain line (CL). Nasion (N), tuberculum (T), basion (B), and dorsum sellae (DS).
The length of the clivus (Figure 1B): The line from the inferior tip of the dorsum sellae to the basion. A short clivus is defined as a clival length less than 32 mm.22
Clivus canal angle (CCA) (23) (Figure 1): The angle formed between the line extending from the top of the dorsum sellae to the basion and the line extending from the basion to the line marking the posterior surface of the odontoid process (i.e., between the most inferodorsal portions of C2 to the most superodorsal part of the dens). The clivus canal angle reflects craniocervical kyphosis that may be correlated with ventral brainstem compression. Craniocervical kyphosis is defined as a CCA angle less than 150°.23
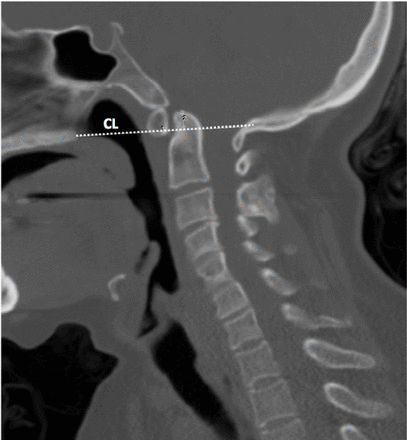
Basilar invagination (Figure 2). The tip of the dens is projecting more than 3 mm above the Chamberlain line.23
Demonstrating the Chamberlain line used to evaluate basilar invagination. CL - Chamberlain line
Other radiologic parameters pertaining to the anatomy the posterior fossa were also evaluated, including the length of the supraocciput (i.e., the line from the opisthion to the internal occipital protuberance; Figure 1C),24 the length of McRae’s line (i.e., the line from the basion to the opisthion; Figure 2),25-27 Twining’s line (i.e., the line between the inferior part of the dorsum sellae to the internal occipital protuberance; Figure 1C),23 and the length of Chamberlain line (i.e., the line between the dorsum of the hard palate and the opisthion; Figure 1C).28
Patients were divided into two groups based on the presence (CVJ+) or absence (CVJ-) of an abnormal finding in one of the included parameters related to the CVJ. To be included in the CVJ+ group, a patient had to have at least one of the following features: short clivus, craniocervical kyphosis, platybasia, or basilar invagination.
Statistical analysis
Statistical analysis was conducted using Statistical Package for the Social Sciences (SPSS) version 22.0 (SPSS Inc., Chicago, IL, USA). Descriptive data are presented as the mean and the standard deviation for numerical variables. The chi square and Fisher’s exact tests were used to compare nominal variables. In addition, a value of p < 0.05 was considered statistically significant.
Results
Patient demographics and clinical data
We identified 210 patients with CM through the imaging database. We excluded patients with CM type 2 (83 patients), an underlying pathology (59 patients), or tonsillar decent less than 5 mm (4 patients). Sixty-four patients were ultimately included for evaluation. The average age was 24±17 years; 28 pediatrics and 36 adult patients. Most (59%) patients were females. The characteristics of the 64 patients were summarized in Table 1.
Characteristics of CM-1 patients with and without craniovertebral junction abnormalities.
Clinical data for 51 patients were available for analysis. Most (82%) patients were symptomatic. The most common symptom at presentation was headache (27%), followed by extremity paresthesia (22%), and gait imbalance (10%). Additional clinical symptoms included neck stiffness, dizziness, and weakness.
Craniovertebral junction evaluation and clinical features
Of the 64 patients, the CVJ+ group included 55 patients. Clinical characteristics of the patients were provided in Table 1. The CVJ- patients were younger than the CVJ+ patients (mean age: 20 years, and 24 years respectively; Table 1); however, this difference was not statistically significant (p = 0.401). The CVJ+ group had significantly more female patients than the CVJ- group (p = 0.012; Table 1). Most patients in the CVJ+ and CVJ- groups were symptomatic (79% and 100%, respectively) without any significant statistical difference (p = 0.4862; Table 1). The most common clinical presentation in the CVJ+ group was headache (30%). However, patients in the CVJ- group commonly presented with dizziness (25%) and with other symptoms; such as seizures, sleep apnea, and facial asymmetry (Table 1).
Decompressions were performed in 18 (33%) patients in the CVJ+ group, compared to 1 (11%) patient within the CVJ- group. This difference was not statistically significant (p = 0.260; Table 2). Among all included patients in this series, 14 (22%) had hydrocephalus. While there were more patients with hydrocephalus in the CVJ+ group, the difference was not statistically significant (p = 0.399; Table 2).
Radiological characteristics of CM-1 patients with and without craniovertebral junction abnormalities.
Description of CVJ radiological parameters
The most frequent CVJ abnormality in our series was platybasia, which accounted for 71% of the CVJ+ group. The second most frequent abnormality was a short clivus in 44% (24 patients), followed by cervical kyphosis in 33%. Among the CVJ+ group, 21 patients were positive for one CVJ abnormality, whereas 34 patients were positive for more than one CVJ abnormity. Patients in the CVJ- group had greater degree of tonsillar decent (Mean: 12.71 mm) than patients within the CVJ+ group (Mean: 11.26 mm) (Table 2). However, this difference was not statistically significant (p = 0.458).
Analysis of syringomyelia
Among all included patients, syringomyelia was detected in 19 patients out of 59 patients for whom MRI of the spine was available for review. Most (93%) of patients with syringomyelia were symptomatic (Table 3). The clinical presentation of patients with syringomyelia included occipital headache (40%), neck stiffness (20%), weakness (27%), and paresthesia (40%). Within the syringomyelia group, the most common CVJ abnormality was platybasia (16 patients), which accounted for 84%, followed cervical kyphosis (53%) and basilar invagination (26%).
Characteristics of CM-1 patients with and without syringomyelia.
All patients in the syringomyelia group in our series had CVJ+ abnormalities, compared to 80% within the non-syringomyelia group (p = 0.045; Table 2).
Within the syringomyelia group, 10 (53%) patients underwent decompression only and 1 (5%) patient underwent decompression and syrinx shunt, compared to 9 (23%) patients in the non-syringomyelia group who were decompressed and 5 (13%) patients who required VP shunt insertion (Table 4). Following the exclusion of hydrocephalus cases (14 cases), syringomyelia was found more frequent in CVJ+ group (33%) compared to (0%) in CVJ- group, but this difference was not statistically significant (p = 0.166).
Radiological characteristics of CM-1 patients with and without syringomyelia.
Discussion
The current study expanded on the findings of previous reports that evaluated CVJ abnormalities in patients with CM1. Various CVJ structural abnormalities have been previously reported in CM1 patients with one or more than one CVJ abnormality.16,29 These abnormalities may include: short clivus, decreased posterior fossa volume, basilar invagination, platybasia, atlas assimilation, and odontoid process retroflexion.13,16,24,29-31 Such CVJ abnormalities can be found in CM1 patients with and without syringomyelia; however, their correlation with syringomyelia is unclear.32,33 Studying one CVJ abnormality might not be sufficient. For instance, a comparative study34 of patients with and without basilar invagination demonstrated that patients with basilar invagination were significantly less likely to have syringomyelia. However, basilar invagination was the only CVJ abnormality included in that study. In comparison, the current study was more inclusive by considering several CVJ abnormalities where syringomyelia was more frequent in CVJ+ group compared to CVJ- group. It is possible that CVJ structural abnormalities compromises CSF flow in the SAS and further contributes to the development of syringomyelia. Understanding such correlation may add to the understanding of CM1 pathophysiology and help early recognition and treatment of syringomyelia to prevent irreversible deficits.7
The pathophysiology of the development of syringomyelia in CM1 remains a subject of debate. However, it is generally believed that the pathophysiology of syringomyelia is explained by an interruption in the CSF flow dynamics at the level of the foramen magnum.6,7,9 Compression of the SAS at the foramen magnum by the cerebellar tonsils has been assumed.6,9 Increased subarachnoid pressure waves subsequently transmit syrinx fluid distally with each cardiac cycle. Subsequently, syrinx formation and progression results.6,7,9 Intradural arachnoid adhesions may further contribute to syrinx development and progression.29 Hence, surgical decompression and duraplasty may reverse the pathophysiological process of syrinx in patients with CM1.7 This finding emphasizes the importance of diagnosing syringomyelia early to reverse the pathophysiology of syrinx and prevent irreversible damage.
In other disease conditions, the presence of CVJ bony abnormalities with an associated hindbrain herniation has been postulated to cause CSF flow disturbance with the result of syringomyelia.35-37 For instance, this phenomenon was reported in cases of osteogenesis imperfecta in which a progressive reduction in the volume of the posterior fossa secondary to invagination was associated with cerebellar tonsillar abnormalities and syringomyelia.36,37 Additionally, mechanical instability at the atlantoaxial joint has been implicated in the pathophysiology of CM1, with surgical fusion been proposed as the appropriate treatment.38,39
The pathophysiology of the association between hydrocephalus and CM1 is a subject of debate.40,41 A raised intracranial pressure caused by hydrocephalus could result in an abnormal tonsillar decent and possibly syringomyelia formation. However, another theory has related the development of hydrocephalus in CM1 patients to the interruption of CSF dynamics in the area of foramen magnum.40,41 The disturbed CSF flow was thought to be secondary to the anatomically smaller posterior fossa.40,41 While the association is a subject of an ongoing research, it is generally accepted that treating hydrocephalus is a priority in patients presenting with hydrocephalus and CM.40-42 In the current study, there was a lack of significant association between abnormalities at the CVJ and syringomyelia following the exclusion of hydrocephalus cases despite more CVJ+ cases in the syringomyelia group. This could be related to the small sample size or to the complex association between hydrocephalus and CM in the presence of abnormalities in the posterior fossa anatomy. This could be further explored in a future study with a larger sample size given such limitation in the present study.
The current study should be interpreted within its existing limitations. The retrospective nature of the study has the possibility of bias. However, bias is less of a concern in the current study because our primary analysis depended on the imaging features of patients with CM for whom a neuroradiologist reviewed the images and was blinded to the clinical data. While features of abnormal structural changes in the CVJ are countless; we included major abnormalities that have been addressed in the literature with clear normal values.
In conclusion, among CM1 patients, syringomyelia was significantly associated with the presence of CVJ abnormalities. However, when hydrocephalus cases were excluded, the association was not significant. Future studies with larger sample size could address the association between CVJ abnormalities and both syringomyelia and hydrocephalus in CM1 patients.
Supplements
* Supplements will be considered for work including proceedings of conferences or subject matter covering an important topic
* Material can be in the form of original work or abstracts.
* Material in supplements will be for the purpose of teaching rather than research.
* The Guest Editor will ensure that the financial cost of production of the supplement is covered.
* Supplements will be distributed with the regular issue of the journal but further copies can be ordered upon request.
* Material will be made available on Saudi Medical Journal website
Footnotes
Disclosure. Authors have no conflict of interests. This study was supported by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia.
- Received March 19, 2020.
- Accepted May 21, 2020.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.