Abstract
Objectives: To explore the clinicopathological features and prognosis of multifocal high-grade gliomas (M-HGGs) with H3F3A mutation in adults.
Methods: Four adult patients with H3F3A-mutant M-HGGs who were treated at our institution from August 2020 to December 2021 were reviewed, including clinical, pathological and radiologic data. A series of 16 adult patients with M-HGGs without H3F3A mutation was used as a comparative group. Progression-free survival (PFS) and overall survival (OS) were compared between the groups using the Kaplan–Meier method.
Results: All patients were IDH wild-type and TERT wild-type, and P53 was overexpressed. A patient with the H3 G34R mutation and 1 of 3 patients with the H3 K27 M mutation had MGMT promoter methylation. The lesions with the H3 G34R mutation were located in the cerebral hemisphere; the lesions with H3 K27 alterations were mainly in the midline structure, and the cerebral hemisphere could also be involved. One patient underwent subtotal resection (STR), and 3 patients underwent biopsy. All patients received radiotherapy, and the median PFS and OS were 9.5 months and 14.5 months, respectively. The clinical outcomes were similar to those of non-H3F3A-mutated M-HGGs patients (median PFS and OS were 7.0 months and 18.0 months, respectively).
Conclusion: We describe the clinicopathological features and outcomes of 4 adult M-HGGs patients with H3F3A mutation, and found this mutation doesn’t appear to have a negative outcome with the administration of current therapies.
In recent years, the mutations of a regulatory histone H3F3A, including H3 K27 M and H3 G34R/V mutations, have been found to be an important genetic driver of high-grade glioma, especially in children and adolescents.1-2 The biological behavior of high-grade glioma with H3F3A mutation often shows a diffuse infiltrative growth pattern. Previous reports revealed that H3 K27 M-mutant glioma commonly occurs in the midline structure,3 while H3 G34-mutant glioma is predominantly found in the supratentorial nonmidline structure.4 The mutation rate of H3 G34 is lower than that of H3 K27 M, and they are mutually exclusive, and exclusive from IDH-mutant cases.4-5 According to the 2021 World Health Organization (WHO) classification of CNS tumors, pediatric-type diffuse high-grade gliomas are named “Diffuse midline glioma, H3 K27-altered” and “Diffuse hemispheric glioma, H3 G34-mutant”.6 However, the H3F3A mutation in adult-type diffuse gliomas is still not fully understood.
Multifocal high-grade gliomas (M-HGGs) are a subtype of malignant glioma with a worse prognosis than solitary high-grade glioma.7-8 M-HGGs with H3F3A mutations are rare. This study retrospectively reviewed the clinicopathological features and outcomes of M-HGGs with H3F3A mutation in adults to gain insight into the understanding of high-grade gliomas with H3F3A mutation.
Methods
We retrospectively analyzed 4 adult patients with H3F3A-mutant M-HGGs who underwent radiotherapy at Xuanwu Hospital, Capital Medical University from August 2020 to December 2021. A series of 16 adult patients with M-HGGs without H3F3A mutation was used as a comparative group. Cases with solitary high-grade glioma were excluded. The patients underwent at least 6 months of postoperative follow-up. The cases were classified according to the 2016 WHO classification of CNS tumors.9 The M-HGGs are defined as 2 or more lesions on magnetic resonance imaging (MRI). The clinical, radiologic and pathological data were reviewed. This study was reviewed and approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University.
The extent of resection was classified as gross total resection (GTR), subtotal resection (STR), and biopsy. Postoperative radiotherapy was performed with the volume-modulated arc therapy (VMAT) technique based on the recommendations for high-grade glioma target delineation in the 2020 version of the National Comprehensive Cancer Network (NCCN) guidelines. Chemotherapy was given using the Stupp protocol.
Histological pathology is the result of hematoxylin and eosin (HE) staining and immunohistochemical staining, and antibodies are routinely detected, including IDH1, P53, Ki-67, and H3 K27 M. H3 G34R/V was partially detected. The molecular data included IDH1/2, TERT, MGMT promoter methylation, and partial H3 G34 or H3 K27 detection. Mutational analysis of IDH1/2, H3 G34 and H3 K27 was performed using polymerase chain reaction and Sanger sequencing. MGMT promoter methylation was assessed by pyrosequencing.
Progression-free survival (PFS) was defined as the time from surgery to progression. Overall survival (OS) was defined as the time from surgery to death from any cause or to the date of the last visit. Follow-up was mainly performed through outpatient and telephone visits. Clinical characteristics were described using descriptive statistics, while survival analysis comprised the median PFS estimation, the median OS estimation, the Kaplan–Meier curve, and the log-rank test using GraphPad Prism.9 A p-value <0.05 was considered statistically significant.
Results
Patient characteristics
The median age of adult patients with the H3F3A-mutated M-HGGs was 36 years old (range, 27-65 years), including 2 males and 2 females (Table 1). Headache was the most common clinical symptom, without seizure. All patients underwent surgery, including 1 with STR and 3 with biopsy. Two patients received concomitant radiochemotherapy, another 2 patients received radiotherapy alone, and one patient received tumor-treating fields after radiotherapy.
- Characteristics of 4 patients with H3F3A-mutant M-HGGs.
Imaging characteristics
The imaging characteristics of H3F3A-mutant M-HGGs are shown in Figure 1-4.1-4 According to the MRI results, three patients had 2 lesions, and the lesions were found on the right side of the brain with enhancement. In another patient, 3 lesions in the bilateral brain showed no enhancement. The location of the lesions in one patient was in the midline structure, that of another patient was in the cerebral hemisphere, and both the cerebral hemisphere and midline structure were involved in another 2 patients.
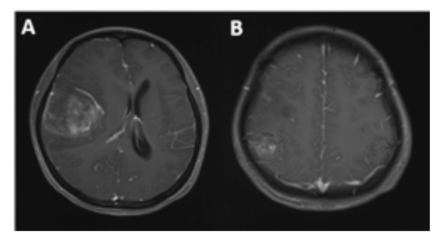
- Radiographic features of case 1. Contrast-enhanced MRI shows 2 lesions in the right frontal-temporal (A) and right parietal lobes (B).
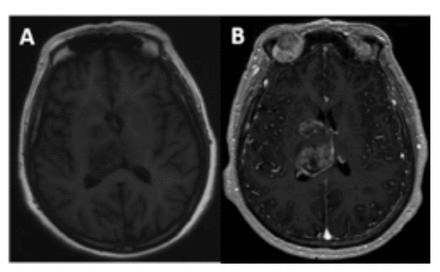
- Radiographic features of case 2. T1-weighted image shows 2 lesions located in the right basal ganglia and right thalamus (A), with obvious inhomogeneous enhancement on enhanced scans (B).
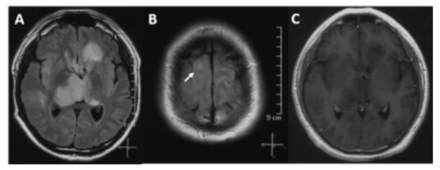
- Radiographic features of case 3. The lesion was unevenly enhanced on contrast-enhanced MRI. The FLAIR image shows multiple lesions in the left frontal lobe, bilateral thalamus (A), and right frontal lobe (B), and the lesions are not enhanced (C).
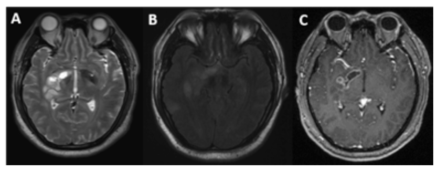
- Radiographic features of case 4. The T2-weighted image shows that the lesion was located in the right basal ganglia, involving the thalamus (A), and the FLAIR image shows that another lesion was located in the right temporal lobe (B). Contrast-enhanced MRI shows that the lesion was enhanced in the right basal ganglia (C).
Pathological features
Histopathological immunohistochemistry showed that P53 was overexpressed in 4 patients, and IDH1 was positive in one patient but negative in next-generation sequencing (NGS). The H3.3 histone test showed that one patient was H3 G34R positive, and 3 patients were H3 K27 M positive. The Ki-67 index was between 15% and 50% in H3 K27-altered patients, and the Ki-67 proliferation rate was up to 80% in H3 G34-mutant patients.
The molecular data are shown in Table 1. None of the 4 patients had IDH mutations or TERT mutations. Two patients had MGMT promoter methylation, including 1 patient with the H3 G34R mutation and 1 patient with the H3 K27 M mutation. The mutational analysis findings of histone H3.3 were consistent with the immunohistochemical results.
Follow-up and outcomes
All patients passed away at the last follow-up. Two patients with H3 K27 alterations had local recurrence; other 2 patients (one with H3 K27 alteration, one with H3 G34R mutation) experienced distant brain progression. The median PFS and OS were 9.5 months and 14.5 months, respectively.
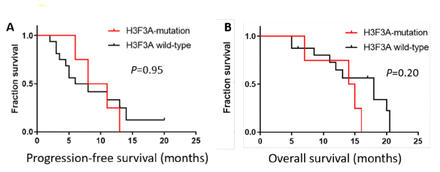
The clinical outcomes of patients with H3F3A-mutant M-HGGs were compared to those of 16 M-HGGs patients without H3F3A mutations treated at the same time. In the control group, 1 patient had an IDH1 mutation, and 4 of 16 patients had MGMT promoter methylation. The median PFS and OS of patients without H3F3A mutation were 7.0 months and 18.0 months, respectively. There was no significant difference between the 2 groups (Figure 5).
- Kaplan–Meier survival curve. Kaplan–Meier survival curves and log-rank tests for PFS (A) and OS (B). The median PFS and OS were 9.5 months and 14.5 months, respectively, in M-HGGs patients with H3F3A mutations. For non-H3F3A-mutated M-HGGs patients, the median PFS and OS were 7.0 months and 18.0 months, respectively. Comparisons between the 2 groups using the log-rank test indicated no significant difference.
Discussion
To date, an increasing number of H3F3A-mutant high-grade gliomas have been reported, yet M-HGGs with H3F3A mutations are rare. It is not clear whether multifocal gliomas represent a unique biological variation or whether multifocal gliomas are an inevitable process in the natural history of high-grade glioma.10 Previous studies have shown that the incidence of M-HGGs is approximately 0.5%-35%,11 but the incidence of M-HGGs with H3F3A mutation is unknown. In this study, we describe the clinicopathological characteristics and outcomes of 4 adult M-HGGs patients with H3F3A mutation, including one with H3 G34R mutation and 3 with H3 K27 alteration.
High-grade glioma with the histone H3 mutation tends to occur in children but also in adolescents or young patients, most of whom are younger than 30 years.12-13 The prognosis of H3F3A-mutant glioma has been reported with varying results in children and adults.3-4,14-16 Vuong et al. showed that the prognosis of H3 K27 M mutation is influenced by patient age and that the pediatric group has a favorable prognosis.12 Tumors with the H3 G34 mutation were consistently located within the cerebral hemisphere and frequently invaded the parietal and temporal lobes.4 Interestingly, H3 K27 M-mutant diffuse midline gliomas in adults mainly involved the thalamus and spinal cord.3 However, in pediatric patients, the brainstem is the most common location of tumors harboring the H3 K27 M mutation.13 In the present case series, the radiological characteristics of adult H3F3A-mutant gliomas are consistent with those reported in previous studies. The lesions of H3 G34R-mutant patients were located in the frontal, parietal and temporal lobes. Three patients with H3 K27 alterations had lesions involving the thalamus, and 2 patients had lesions affecting the basal ganglia.
As previously reported, the H3F3A mutation was exclusive to IDH mutations and TERT promoter mutations.5,17 H3 G34-mutant gliomas were associated with MGMT promoter methylation in most patients, which is also one of the reasons for its better prognosis.15 Our data showed that 4 patients were IDH wild-type and TERT wild-type; P53 was overexpressed in all patients; and the patient with the H3 G34R mutation and 1 of 3 patients with the H3 K27 M mutation had MGMT promoter methylation. The PFS was better than that in patients with MGMT promoter unmethylation.
Although case reports of M-HGGs with H3F3A mutation have been published, few studies have reported the outcome. Lim et al18 and Wang Q et al19 reported one patient with multifocal H3 K27 M-mutant diffuse midline glioma respectively, but the final survival outcome was not obtained.18-19 Yekula et al20 reported one adult H3 K27 M-mutant diffuse midline glioma with a gliomatosis cerebri growth pattern, and the OS was 8 months. Schulte et al reported H3 K27 M-mutant multifocal gliomas in 2 of 60 adult patients with diffuse midline glioma. The median PFS was 9.6 months and the OS was 27.6 months in this adult cohort.21 Vettermann et al22 reported 2 patients with H3 G34-mutant multifocal gliomas without an outcome or molecular profile.22 In our series, the median PFS and OS of all 4 patients were 9.5 months and 14.5 months, respectively. The clinical outcomes were similar to those of non-H3F3A-mutated M-HGGs patients.
At present, there is no standard treatment for M-HGGs, and the commonly used treatment modalities include surgery, radiotherapy, and chemotherapy. There were 3 H3 K27-altered patients with biopsy and one H3 G34-mutant patient with STR; all 4 patients received radiotherapy, and 2 patients received temozolomide during this study. The recurrence pattern of M-HGGs in adults with H3F3A mutation was similar to that of high-grade glioma: 2 patients had local recurrence, and 2 patients had distant brain lesions with diffuse leptomeningeal enhancement. There have also been reports of high-grade glioma with the H3F3A mutation with extraneural metastasis.23-25 The most common sites of distant metastasis are the osseous and peritoneal regions. Mohiuddin et al. reported three cases of high-grade glioma with H3F3A mutation and diffuse extraneural dissemination in young adulthood (age range: 10-25 years).25 However, extraneural metastasis in adults with H3F3A-mutant glioma has not been reported. The patient with the H3 G34R mutation in our study experienced gastrointestinal bleeding after recurrence of the disease, but an abdominal computed tomography (CT) scan showed no obvious abnormalities, and the patient died less than a month after the onset of intestinal bleeding.
The main limitation of this study is the small number of patients due to the rarity of M-HGGs with H3F3A mutations. Moreover, the patients did not receive salvage treatment, such as reirradiation or systemic treatment, after disease progression because of coronavirus disease 2019 pandemic and personal reasons. This may affected the overall prognosis of the patients to some extent, and the patients died soon after recurrence.
In Conclusion, The H3F3A-mutant glioma mainly affects the pediatric population but can also occur in adults. In this study, we analyzed a case series of M-HGGs with H3F3A mutation in adults, including clinical, radiological, pathology, and outcome data. This disease entity may rarely be present in the clinic and has a complicated, multifactorial etiology that has not yet been fully described. Even so, current treatments may lead to a satisfactory prognosis. More clinical studies are needed to further investigate the clinicopathological characteristics and prognosis of H3F3A-mutant M-HGGs in adults.
Acknowledgment
We acknowledge the work of our colleagues at the Radiology Department in offering the original images and data related to this article. We acknowledge the neurosurgeons for their special contributions to these cases.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received July 8, 2022.
- Accepted November 23, 2022.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.