Abstract
Objectives: To review the experience of 2 tertiary centers in Saudi Arabia with intracranial hypertension (IH) in the pediatric population.
Methods: We retrospectively reviewed and analyzed pediatric patients diagnosed with IH from June 2002 to May 2017 in 2 institutes.
Results: We identified 53 patients (30 females and 23 males) with a mean age of 7 years at the time of presentation. Among them, 41 patients were younger than 12 years, and 12 were older. Obese and overweight patients constituted 27.00% (n = 14) of all cases, 8 (66.7%) of whom were older than 12 years. The most common presenting feature was papilledema followed by headache. Vitamin D deficiency, which constituted the most common associated condition, was identified in 12 (22.6%) patients. Acetazolamide was the treatment option in 98.11% of patients, and only 5.7% underwent surgical interventions. The length of follow-up ranged from 6 months to 8 years.
Conclusion: Intracranial hypertension is rare in children and commonly seen in overweight females older than 12 years similar to adults. Patients younger than 12 years tend to develop secondary IH. More studies are needed to characterize the clinical presentation and guide the management plan.
Intracranial hypertension (IH) is rarely reported in children. It is characterized by increased intracranial pressure (ICP) without any evidence of underlying brain pathology, structural abnormalities, hydrocephalus, or any abnormal meningeal enhancement.1 The incidence of IH differs from region to region due to variations in the prevalence of obesity and other secondary causes. The annual incidence of IH in children is 0.9 per 100,000 in the United States,2 0.5 per 100,000 in Germany,3 0.6 per 100,000 in Nova Scotia and Prince Edward Island in Eastern Canada,4 and 1.2 per 100,000 in Croatia.5 A study carried out in Oman estimated the incidence of IH to be 1.9 per 100,000 in children below 15 years of age; with it being higher in female children.6 The present study aimed to review the clinical presentation, possible aetiological factors, diagnosis, management, and outcomes in children with IH in 2 tertiary institutes in Saudi Arabia.
Methods
After approval of the study proposal by the Research Ethics Committee at King Saud University, and King Abdullah Research Center, we did a retrospective review of all pediatric patients diagnosed with IH between June 2002 to May 2017 at King Saud University Medical City and King Abdullah Children Specialist Hospital, National Guard Health Affairs. Patients with identified aetiologies were classified as having secondary IH (SIH), and patients without identified aetiologies were classified as having idiopathic or primary IH (PIH). The following parameters were recorded: patients’ characteristics, age at the time of presentation, presenting symptoms and signs, associated conditions, diagnostic procedures, brain images, treatment, and outcome. IH was defined based on the modified Dandy criteria.7 The inclusion criteria were: patients aged between 6 months and 18 years, normal brain magnetic resonance imaging (MRI) and/or computed tomographic (CT) scan, a cerebrospinal fluid (CSF) opening pressure greater than 250 mm H2O with normal CSF analysis, bilateral papilledema, and intact neurologic examination except for isolated abducent, trochlear or oculomotor paresis. We excluded patients who had abnormal CSF study results, normal or undocumented ICP pressure, brain imaging showing structural abnormalities, and abnormal neurological examination other than sixth nerve palsy or papilledema.
We identified a total of 64 cases; 11 were excluded for missing data, lack of documentation, or inability to meet the inclusion criteria, thus resulting in the inclusion of a final 53 patients. We classified the patients as either prepubertal or pubertal. The use of secondary sexual characteristics is often the proper method to classify children as either pubertal or prepubertal. However, due to the retrospective nature of this study, we instead chose an average age. Therefore, patients younger than 12 years were considered prepubertal, while patients older than 12 years were considered pubertal. Based on their body mass index (BMI), we further classified the patients into three groups: obese, overweight, or normal body weight. A child was described as obese if the BMI was more than 30 and overweight if the BMI ranged between 25 and 29.9.
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 19 (IBM Corp., Armonk, N.Y., USA).
Results
A total of 53 children with IH were analyzed in this study. The mean age of the patients at presentation was 7.7 years, ranging from 8 months to 16 years. Twenty-three children were male and thirty were female. Most individuals (n=36 [69.2%]) had normal weight, 7 (13.5%) were overweight, and 7 (13.5%) were obese. The demographics of the participants are shown in Table 1. In the present study, 12 patients were 12 years and older (pubertal group) and 41 patients were younger than 12 years (prepubertal group). The mean age at presentation in the pubertal group was 13.66±1.66 years (range=12-16 years) and 5.9±3.38 years (range=8 months -11 years) in the prepubertal group. The percentage of females in the prepubertal group was 52.63% and pubertal group was 66.66%. Obese and overweight patients constituted 27.00% (n=14) of all cases, and 66.7% (n=8) of those in the pubertal group (Table 1). Compared to those in the pubertal group, most patients in the prepubertal group (n=34 [85%]) were underweight or had normal weight. The prepubertal group was further categorized into three groups based on age, those less than one year old at diagnosis (n=3 [7.3%]), those between 1 and 6 years old (n=20 [48.8%]), and those older than 6 years (n=18 [43.9%]).
Demographics of the participants.
The most common clinical feature was papilledema (n=52 [98.1%]) followed by headache (n=42 [79.2%]). Papilledema was asymptomatic in 16 (30.2%) patients. Other common symptoms included visual problems such as photophobia, diplopia, and blurred vision. Neurologic examination was reported as normal in 29 (54.71%) patients, and sixth nerve palsy was present in 4 (7.54%) patients. All the patients had an insignificant perinatal history, except 2 who were born prematurely at 30 and 34 weeks of gestation. The clinical features of the patients are shown in Table 1. Figure 1 & 2 demonstrates papilledema in some of the patients.
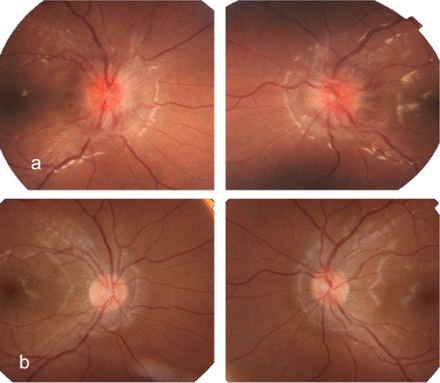
Fundus photographs of a 9-year-old girl showing her optic nerves. A) At presentation, the right and left optic nerves show papilledema. B) The right and left optic discs with resolving papilledema a few months later after medical treatment.
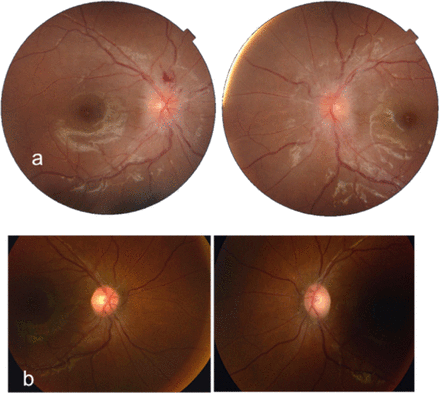
Fundus photographs of a 12-year-old girl showing her optic nerves. A) At presentation, the right and left optic nerves show papilledema with peripapillary flame-shaped hemorrhages seen in the right eye indicating an acute process. B) Flat right and left optic discs a few months after medical treatment.
Out of the 53 patients, 13 patients had PIH (idiopathic) and 40 patients had SIH (Table 2). There were no significant differences in gender, age at presentation, or BMI between the PIH and SIH patients. Interestingly, 16 (44.4%) patients with SIH required additional lumbar puncture (LP). In terms of outcome, the recurrence in the SIH group was high (n=6 [19.4%]), whereas only one patient had a recurrence in the PIH group. At the time of presentation, majority of the patients (n=47 [88.7%]) were not on any medications except for 2 (3.77%) who were on carbamazepine, one (1.9%) who received growth hormone therapy, one (1.9%) on steroids and cyclosporine, and one (1.9%) on methotrexate (Table 2). Lumbar puncture was carried out in all cases. Opening pressure was high in all patients. Cerebrospinal fluid analysis was normal in all patients. All patients underwent neuroimaging studies, including CT and/or MRI brain scans. Four (7.5%) patients had craniosynostosis. Magnetic resonance venography (MRV) had been performed in 37 (69.8%) patients. Sagittal dural sinus thrombosis was evident in one patient. The MRI showed typical IH features in more than half of the patients 36 (67.9%). Figure 3demonstrates the findings in one of the patients. Further laboratory tests were performed in most patients and included the thyroid function test, antinuclear antibody test, and C3, C4 and 25 (OH) vitamin D levels. All these tests showed normal results except for 25 (OH) vitamin D levels, which were low in 12 (22.6%) patients.
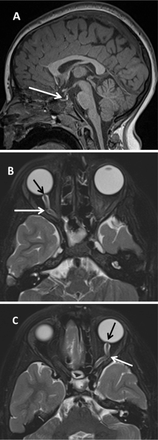
MRI Brain and orbit for a 10-year-old girl with primary IH. MRI sagittal T1WI brain. A) showing partial empty sella (white arrow). Axial T2 fat saturation for orbits, B&C) showing flattening of posterior sclera and prominence of the optic nerve head (black arrow) as well as tortuosity of optic nerve and prominent perioptic nerve sheath (white arrow).
Associated conditions and medications (Secondary Intracranial Hypertension).
The majority of patients (n=52 [98.1%]) were treated with acetazolamide as first-line therapy. Only one patient was treated with topiramate alone. Out of the 52 patients who received acetazolamide, 29 (54.71%) did not require further treatment. Most patients (n=50 [94.3%]) did not require surgical intervention, and only 3 (5.7%) patients underwent surgical treatment (lumboperitoneal shunt). According to the long term outcome, there were no relapses in 46 (86.8%) patients. None of our patients had optic nerve fenestration. The surgical treatment and recurrence are summarized in Table 3. The length of follow-up ranged from 6 months to 8 years.
Surgical treatment and recurrence.
Discussion
The IH is typically present in overweight women; however, it can occur at any age. Unlike in adults, several studies have found that IH in prepubertal children is less commonly associated with obesity, and has no female predominance.8,9 On the other hand, IH in postpubertal children is usually similar to that in adults, and is associated with overweight and commonly occurs in women.10 In our study, obese and overweight patients constituted 27.00% (n=14) of all cases, a majority of whom were pubertal. This classification is rarely done in pediatric IH cases reported in the literature.
As the name implies, the pathophysiology of IH is not fully understood. There are several proposed hypotheses such as increased CSF production, decreased CSF outflow, increase in cerebral blood volume, increase in water content, venous obstruction, chronic inflammation, and metabolic causes.9 Obesity is an important risk factor for IH in postpubertal females.11 However, the absence of obesity and sex predilection in some prepubertal children and adolescents with IH suggests a different pathogenesis in these patients. Hence, a secondary cause should be carefully investigated and ruled out before making a diagnosis.4,11,12
Additionally, IH can also be secondary to an underlying medical condition or use of certain medications. In a recent study of IH in the pediatric population, 30% of the cases were found to have a secondary cause.11 Several factors have been associated with IH in pediatric patients. These include hyperparathyroidism, thyroid replacement therapy, treatment with recombinant human growth hormone, Chiari malformation, meningitis, hydrocephalus, craniosynostosis, traumatic brain injury, superior sagittal sinus thrombosis, leukemia, congestive heart failure, renal failure, and kidney transplantation.11,12 In a recent study, vitamin D deficiency was found in 26.3% of pediatric IH patients, with none of them having hypocalcemia or signs and symptoms of rickets.13 In our study, 12 (22.6%) cases were found to be associated with vitamin D deficiency. The exact pathophysiology of increased intracranial pressure in relation to vitamin D deficiency is not fully known. In the present study, one case of hypoparathyroidism-retardation-dysmorphism syndrome, also known as Middle east syndrome or Sanjad-Sakati syndrome (OMIM # 241410), had IH possibly resulting from the associated hypoparathyroidism, a recognized risk factor of IH.14
The most common clinical presentation in children is headache.11,15,16 Other clinical features include nausea and vomiting, blurred vision, and double vision. The neurological examination of children with IH is usually normal except for papilledema, decreased visual acuity, visual fields defects, neck stiffness, and sixth nerve palsy. Although papilledema is an important sign in children with IH, the absence of papilledema does not rule out IH.17,18 A previous study reported that papilledema was absent in 48% of the patients. Interestingly, one of our patients (1.9%) did not have papilledema at the time of presentation.
Imaging studies are essential in the diagnosis of this condition. A CT scan should be done first, and if the findings are normal, then a lumbar puncture with opening pressure should be performed.19 Further tests should be considered to rule out secondary causes. Brain MRI is used to rule out intraparenchymal lesions, abnormal meningeal enhancement, or hydrocephalus.20 The MRV is used to rule out cerebral venous sinus thrombosis.20 MRI findings suggestive of IH in children include: posterior globe flattening, intraocular protrusion of the optic nerve, horizontal tortuosity of the optic nerve, optic nerve sheath enlargement, and decreased size of the pituitary gland.21,22 Lumbar puncture is indicated to measure the CSF opening pressure and to exclude meningitis.1,23 Diagnosis of IH is made according to the modified Dandy criteria.1 However, new criteria in children involving a specific CSF opening pressure have been introduced into the literature by Friedman and his colleagues.24 The criteria also take into consideration the diagnosis of IH when the presentation is not clear or atypical. In this study, SIH was more common than PIH, especially in children under 12 years. Moreover, IH was strongly associated with syndromes such as craniosynostosis and Down syndrome or other medical problems such as vitamin D deficiency. Different treatment modalities have been indicated;25,26 however, the selection of medical, surgical, or combined treatments relies on the severity of the visible signs and symptoms. Medical treatment, including carbonic anhydrase inhibitors, which have been used to lower ICP, is initially indicated. The most commonly used carbonic anhydrase inhibitor is acetazolamide, which is usually used as first-line therapy.1 Its use has been thoroughly studied and is thought to decrease production of CSF in the choroid plexus. However, the use of topiramate has not been as thoroughly examined as a monotherapy agent for IH in adults or children. Topiramate is used for the treatment of IH through its inhibitory effect on carbonic anhydrase enzyme, which also causes mixed renal tubular acidosis. Moreover, its side effect of appetite suppression makes it an effective adjunct agent for individuals with obesity.27 In our study, medical treatment was effective in most cases (86.8%), with four cases being treated by topiramate.
Additionally, steroids can be used to reduce intracranial pressure rapidly. Nonetheless, it is only recommended for short-term use in severe cases where surgical intervention is not immediately possible.28 In our study, steroids were only used in 4 cases.
Surgical treatment in the form of optic nerve sheath fenestration (ONSF) and CSF diversion is indicated when medical treatment fails to lower ICP, or when the visual impairment is deteriorating.16 The most commonly used CSF diversion procedures are lumboperitoneal and ventriculoperitoneal shunting, with lumboperitoneal shunting being reported as the most successful in relieving symptoms.16 Only three patients in this study required surgical management in the form of lumboperitoneal shunting. ONSF is indicated for acute, severe, or progressive vision loss despite maximum medical treatment.16 None of our patients underwent ONSF.
Additionally, lifestyle modifications such as weight reduction in overweight and obese patients can decrease the symptoms associated with IH in adults. However, in the presence of visual deterioration, weight loss alone is insufficient to reduce ICP within the appropriate time.1 Interestingly, bariatric surgery can be considered in morbidly obese IH children who failed multiple trials of weight loss, with favorable outcome.29,30 During the follow-up period, 7 (13.2%) cases had a recurrence. Previous studies have reported an IH recurrence rate in children of up to 18-22%.31,32 Although visual loss in pediatric patients with IH has been previously reported, permanent vision loss in is usually rare.31 In our study, most patients did not have any signs or symptoms of visual deterioration.
Study limitation
This study has several limitations, including its retrospective nature and small sample size.
In conclusion, IH in children is rare and differs from that in adults. Unlike in adults, IH in pre-pubertal children is less commonly associated with obesity, has no female predominance, and more commonly manifests as SIH. On the other hand, IH in postpubertal children is usually similar to that in adults, and is associated with overweightness as well as female predominance. Our study adds to the body of evidence by reporting on our institutions’ experience with IH in the pediatric population.
Authorship entitlement
Excerpts from the Uniform Requirements for Manuscripts Submitted to Biomedical Journals updated November 2003.
Available from www.icmje.org
The international Committee of Medical Journal Editors has recommended the following criteria for authorship; these criteria are still appropriate for those journals that distinguish authors from other contributors.
Authorship credit should be based on 1) substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; 2) intellectual content; and 3) final approval of the version to be published. Authors should meet conditions 1, 2, and 3.
Acquisition of funding, collection of data, or general supervision of the research group, alone, does not justify authorship.
An author should be prepared to explain the order in which authors are listed.
Acknowledgments
M. A. S. was supported by Researchers Supporting Project number (RSP-2019/38), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received June 12, 2019.
- Accepted August 13, 2019.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.