Abstract
Objective: To evaluate the effects of Catechin (CAT) on memory acquisition and retrieval in the animal model of sporadic alzheimer’s disease (sAD) induced by intracerebroventricular (icv) injection of streptozotocin (STZ) in passive avoidance memory test.
Methods: Thirty adult rats were divided into 5 experimental groups (n=6). Animals were treated by icv saline/STZ (3 mg/kg) injection at day one and 3 after cannulation. The STZ+CAT group received 40 mg/kg CAT by daily gavages for 10 days, after icv STZ treatment and before training. The step-through latency (STL) and time spent in the dark compartment (TDC) were evaluated to examine the memory acquisition and retrieval. All tests were performed in Qom University of Medical Sciences, Qom, Iran, from April to December 2013.
Results: The STZ treatment significantly decreased STL and increased the number of entries to the dark compartment on the training day. It also increased TDC, on day one and 7 after training. Pre-training gavage of CAT reversed the STL significantly (p=0.027). The CAT treatment also decreased the TDC in both early and late retrieval, in respect to STZ group.
Conclusion: This data suggests that CAT as an antioxidant could improve both memory acquisition and retrieval in the animal model of sAD.
According to reports in 2012, there are more than 35 million people living with dementia worldwide.1 Alzheimer’s disease (AD), which is characterized by amyloid (Aβ) plaque accumulation, is the most common forms of dementia, and results in memory loss and cognitive impairment.2 The pathological features of AD include Aβ peptide misfolding that form neurotic plaques on the neurons and intracellular neurofibrillary tangles composed of hyperphosphorylated tau protein.3 There are many reports indicating elevated oxidative stress in the brain of patients with mild cognitive impairment or AD.4 Oxidative stress may be the first feature in the brain of AD patients,5,6 which appears even before Aβ deposition.7,8 Transgenic mice bearing a mutant amyloid precursor protein (APP) similarly showed oxidative stress before Aβ deposition.9 The Aβ plaque formation is suggested to be an effort by the cell to protect itself against oxidative stress.10 The Aβ metabolism increases reactive oxygen species (ROS) and decreases adenosine triphosphate production in mitochondria.11 The secretion, oligomerization, and aggregation of Aβ is the result of its ROS sequestering activity and leads to destruction of cellular integrity.12 Other consequences of cellular oxidative damage include cell cycle aberration and tau hyperphosphorylation, leading to the formation of neurofibrillary tangles.13
Polyphenolic flavonoids are neuroprotective against oxidative stress14 and possess potent radical scavenging15 and anti-inflammatory activities.16 Catechin (CAT) protects cultured mesencephalic neurons against 6-hydroxydopamine treatment,17 and also prevents primary hippocampal neurons from Aβ toxicity.18 Epicatechin gallate and epigallocatechin gallate was found to increase the activity of oxygen radical species-metabolizing enzymes, superoxide dismutase and catalase, in mouse striatum.19 The CAT is also capable of inhibiting lipid peroxidation induced by iron ascorbate in brain mitochondrial membranes.20 Evidence suggests that damages caused by Aβ can be undermined by antioxidants such as vitamin E21 or polyphenols.22 There are reports that CAT is more effective than vitamin E and C for the destruction of free radicals.15 So these antioxidants could be the major candidates for the prevention and treatment of AD. In this study, we evaluated the effects of CAT as a potent antioxidant on memory acquisition in the animal model of sporadic AD (sAD) induced by intracerebroventricular (icv) injection of streptozotocin (STZ) in passive avoidance memory test.
Methods
Adult male Wistar rats (250–300 g) were kept in temperature controlled (22±2°C), 12-12 hour light-dark cycle rooms with ad libitum access to food and water. All studies were performed in the Qom University of Medical Sciences, Qom, Iran from April to December 2013 and in accordance with the ethical guidelines set by the Ethical Committee of Qom University of Medical Sciences, which is based solely on the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Rats were anesthetized with ketamine (80 mg/kg, intraperitoneal [ip]) and xylazine (20 mg/kg, ip) and were bilaterally implanted with cannulae (23-gauge) placed in the lateral ventricles; (anteroposterior: −0.8 mm from Bregma, mediolateral: ±1.5 mm and dorsoventricular: −2.6 mm from the skull surface) according to the atlas of Paxinos.23
Thirty rats were divided into 5 experimental groups (n=6), namely; Intact, sham, CAT, STZ, and STZ+CAT. The sham group received 2 intracerebroventricular (icv) injection (5 µl/site) of artificial cerebrospinal fluid (147 mM sodium chloride; 2.9 mM potassium chloride; 1.6 mM magnesium chloride, 1.7 mM calcium chloride, and 2.2 mM dextrose), while the STZ group received 3 mg/Kg STZ on day one and 3 after surgery. The CAT group received 40 mg/kg CAT by daily gavages for 10 days before the training day. The dose of CAT is reported to prevent oxidative stress and is capable of reversing oxidative markers in the liver and kidney.24 The CAT+STZ group received CAT, after the first STZ injection. In all groups the training began at day 11 after STZ injection. Memory acquisition and retrieval were examined by passive avoidance test as described elsewhere.25 Shortly; the step-through passive avoidance apparatus consisted of a light and a dark chamber (30 cm×20 cm×20 cm each). The floor of the dark chamber consisted of 2 mm thin, electrified, stainless steel rods. Animals were allowed to explore each chamber for 30 seconds. On the training day, rats were placed in the lighted chamber and the crossover latency was recorded. After entrance a shock (50Hz, 0.5 mA, 2 seconds) was delivered. After 5 minutes the immediate memory was tested and step-through latency (STL) and the number of entries into the dark compartment were measured. Retention tests were performed on day one and 7 after training and time-spent in the dark compartment (TDC) was recorded, up to 300 seconds. For histological examination, 100 µm thick sections were taken and cannulae and injection traces were examined for each side with light microscopy. The recorded time courses of STL and TDC were analyzed, using the Statistical Package for the Social Sciences, Version 20, (IBM Software Group, Illinois, Chicago, United States of America), by one-way analysis of variances, followed by least significant difference post hoc test.
Results
Effect of icv STZ on memory acquisition in passive avoidance task
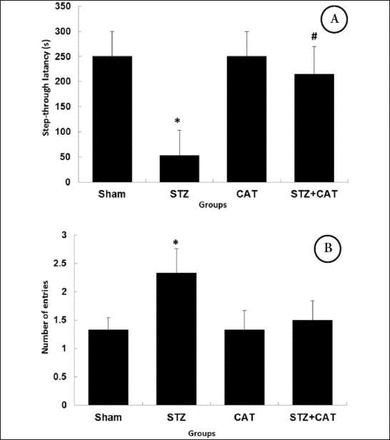
The sham operated rats did not show any significant difference with the naive animals in STL on the training day (T-test). On the training day, as seen in Figure 1A, the mean latencies to enter the dark compartment were significantly shorter in icv STZ treated rats (53.7±49.3 seconds) than in sham-operated ones (250.8±49.1 seconds). The number of trials to acquisition showed also a significant difference between sham-operated (1.34±0.2) and icv STZ treated rats (2.34±0.4) in the acquisition phase. The cut off time was 300 seconds (Figure 1B).
Effects of STZ treatment and pre-training CAT gavage on STL during acquisition phase on the training day A) and on the number of trials to acquisition B). Values are expressed as mean±SEM. *p=0.041 versus Sham and #p=0.027 versus STZ. (n=6, ANOVA multiple group comparison, LSD’s post hoc). STL - Step-through latencies, STZ - streptozotocin treated group, Sham - saline treated, CAT - catechin gavage, and STZ+CAT- streptozotocin treated with catechin gavage, SEM - standard error of the mean, ANOVA - analysis of variance, LSD - least significant difference.
Pre-training Catechin gavage reversed the STL reduction in icv STZ treated rats
Data analysis revealed that daily CAT gavage (40 mg/kg) for 10 days could reverse the reduced STL in icv STZ treated rats (Figure 1A). The STL in the CAT+STZ group was 214±54.9, which is significantly increased (p<0.05) in comparison to icv STZ treated rats (53.7±49.3) and has no significant difference with sham operated rats. Figure 1B shows that although the number of entries was decreased in STZ+CAT group (1.5±0.34), it was not statistically significant. Catechin gavage did not exert any significant effect on memory acquisition in naïve or sham-operated rats, leaving the STL and the number of entries on the acquisition day, unchanged.
Effect of icv STZ treatment on early and late memory retrieval in rats
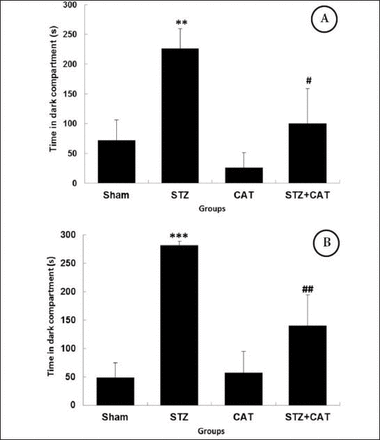
One day after training, icv STZ treated animals spent significantly (p<0.01) longer time (226.2±33 seconds) in the dark compartment as compared with the sham-operated group (71.8±34.6 seconds), which is shown on Figure 2A. The same trend in memory retrieval was true even 7 days after training. The TDC for STZ treated rats was 281.8±6.6 seconds while the sham-operated rats spent just 49.2±26 seconds in the dark compartment (Figure 2B), which even more significantly (p<0.001) indicates the reduction of retrieval 7 days after training in icv STZ rats.
Passive avoidance early and late retrieval as time spent in the dark compartment (TDC) on day one A), and day 7 B) after training. The TDC is indicated in seconds, as mean±SEM. **p=0.009, ***p=0.000 versus sham and #p=0.031, #p=0.006 versus STZ (n=6, ANOVA, post hoc: LSD). STZ - streptozotocin treated group, Sham - saline treated, CAT - Catechin gavage, and STZ+CAT - streptozotocin treated with catechin gavage SEM - standard error of the mean, ANOVA - analysis of variance, LSD - least significant difference.
Effect of pre-training Catechin gavage on early and late memory retrieval in icv STZ treated rats
Pre-training gavage of CAT could prevent the increase in TDC on the first retrieval day, significantly (p=0.041). The TDC was reversed in the STZ+CAT rats (100.6±58.6 seconds) in comparison to the STZ ones (226.2±33 seconds). Even on the seventh day of retrieval, the TDC was less (p=0.006) in the CAT+STZ group (140.3±54.2), while the icv STZ rats remained 281.8±6.6 seconds in the dark compartment on the late retrieval day (Figure 2B).
Discussion
The administration of icv STZ is characterized by progressive deterioration of memory, cerebral glucose and energy metabolism26 and leads to cognitive dysfunction.27 Our results were similar to other studies28 and showed memory impairment after 2 sets of STZ injections. It was previously shown that insulin reduces tau phosphorylation by inhibition of GSK-3β via the PI3-K pathway.29 Therefore, disturbance in the insulin signaling cascade leads to an increase in tau hyperphosphorylation potentiating the formation of NFT.30 Li, et al31 showed that oral administration of green tea catechin for 6 months could prevent age-related spatial learning and memory decline of mice in the Morris water maze. The results of our study showed that pre-training CAT gavage (40 mg/Kg) even for 10 days could improve both the acquisition and retrieval of memory in the passive avoidance task. This is the same dose, which is shown to be able to reduce lipid peroxidation and H2O2 generation in the liver and kidney.24 Prolonged CAT administration prevented either age-related reductions of postsynaptic density-95 proteins and Ca2+/calmodulin-dependent protein kinase II, suggesting that synaptic structural changes may be also involved, in its mechanism of action.31
There are reports indicating oxidative stress as the main cause of AD, occurring prior to Aβ formation.32 Free radicals can have damaging effects directly on the cell, ultimately leading to apoptotic cell death.33 An initial free radical-induced injury would exacerbate a vicious cycle in which amyloidogenic processing of APP would be further enhanced, generating more Aβ that in turn would cause more oxidative stress.34 The Aβ fraction 25-35 may cause lipid peroxidation and ROS formation and lead to neurotoxicity, but catechin pre-treatment was able to decrease the oxidation process and improve memory skills, significantly.35 The CAT has been found to be one of the most powerful ROS scavengers between different members of the different classes of flavonoids.36 It has also been observed that antioxidative enzymes are induced by CAT intake37 and that the antioxidative capacity of plasma is increased by repeated ingestion of green tea.38 These antioxidative defense systems also prevent oxidative damage in the brain. The main limitation of this study was the lack of evaluation of the oxidative markers, which could further clarify the pathogenesis of STZ induced dementia, and elucidate the role of CAT in the improvement process of memory, which could be assessed in future studies.
In conclusion, CAT, like some other antioxidants, could exert neuroprotective effects and prevent memory deficits, probably through reduction of oxidative stress. Although CAT could reverse the STZ induced impairment in learning and memory, both in the training and retrieval tests, but it did not exert any improving effect on intact animals, supporting the idea that CAT is exerting protective effect against oxidative processes.
Footnotes
Disclosure
The authors declare no conflicting interests, support or funding from any drug company.
- Received July 3, 2014.
- Accepted April 27, 2015.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.